Abstract: Safety Considerations in the Generation of Clinical Grade Autologous IPS Cell Lines

Holly Anderson
- Post author:admin
- Post published:April 12, 2019
- Post category:Technologist Winner

Abstract: Safety Considerations in the Generation of Clinical Grade Autologous IPS Cell Lines

Queenie Jang, BSc (Pharmacy), MBA
Chief Executive Officer
Canada
Over the last year, ISCT has focused its investments on building a home for workforce development within the DNA of ISCT. Working with our expansive network of global key opinion leaders and subject matter experts, we have made significant investments to expand our educational offerings. From online resources to practical in-person programs, ISCT members now have access to a wide array of supports.
In recent years, the Cell and Gene Therapy (CGT) field has seen exponential growth, which has outpaced the rate at which new professionals enter. As result, we have witnessed major skill and labor shortages affecting the entirety of the field. Identifying this as a critical issue to the advancement of the sector, ISCT is motivated to be at the forefront of addressing this issue. We see it as our duty to Support the driven and diverse group of regulatory, commercial, and academic professionals and remove barriers and open doors for newcomers entering the field.
Through forming strategic partnerships with outside organizations such as CMaT ANd&AT and Universidad de Granda, ISCT has successfully developed and globally deployed a number of workforce programs spanning translational research to good manufacturing practices and regulatory understanding. 2022 marked not only the launch of the online workforce development in biomanufacturing course in partnership with CMAT but also the decision to make this a recurring program offered four times annually due to the critical need and demand for skilled personnel. Through our partnership with ANd&AT and Universidad de Granda, ISCT, was proud to awarded three European ISCT members full-tuition scholarships to enrol in the Master in Manufacturing of Advanced Therapy Medicinal Products (ATMPs).
Taking a step further to demonstrate ISCT’s unwavering commitment to helping bridge the labour gap in the market, alongside our dedicated head office liaisons, ISCT has formed a specialist workforce development committee. This committee, in collaboration with our global network of subject matter experts, will design training programs created by ISCT members for ISCT members that are tailored to the specific needs of professionals based on their region, area of focus and skill level.
Understanding the criticality of Early Stage Professionals (ESP) for the continued growth of the CGT sector, ISCT has made a deliberate decision to expand and invest in our ESP resources, programs and committees. ESPs currently make up a third of our overall global membership, and we are delighted to see these members becoming more active and engaged in the society. Our ESP Mentoring Program and our ESP Leadership Development Program reached record numbers of participants in 2022, allowing us to connect ESPs with our global CGT network and provide them with practical experience and connections that will last a lifetime. If the cell and gene sector is to continue to grow at the current rapid pace, we must continue to support ESPs and provide them with the tools they need to succeed and excel in their careers.
With workforce development being the greatest challenge that lies ahead for cell and gene therapies, ISCT is proud to champion and spearhead these initiatives. ISCT members, no matter what stage of their professional development, now have more access than ever to the key knowledge and networks that they need to become an effective part of the workforce. We will continue to work collaboratively with our all our stakeholders to develop paths and open doors for both interested professionals and newcomers alike.
Motivated to be a part of the solution and support the continued and sustainable growth of the cell and gene therapy sector, ISCT is spearheading efforts in workforce development, training, and education. In 2022 ISCT was a key player in driving the development and deployment of programs and initiatives available not only to ISCT members but also to the wider CGT community.
In 2022, ISCT successfully launched two new training programs to address critical skills gaps in manufacturing, partnering with the Andalusian Network for the Design and Translation of Advanced Therapies (ANd&tAT) to offer three full-tuition scholarships to ISCT members in Europe. While in parallel, partnering with CMaT, the NSF Engineering Research Center for Cell Manufacturing Technologies, to deliver the first edition of the online program for biomanufacturing.
Recognizing that a third of ISCT members are early-stage professionals, ISCT invested significant resources in expanding the global ESP mentoring and leadership development programs. For the wider global ISCT community, the society also made significant efforts to expand its digital resources for not only facilitating topical sessions but also encouraging cross-collaboration with regional committees.

Bruce Levine, PhD
ISCT Past President (2020 – 2022)
United States
“My passion for ISCT stems from the fact that in my career, no other society has played as integral a role in my professional development.“
Patrick Hanley
ISCT Representative to NASEM
Childrens National Hospital
United States


Meagan Pasternak, BA
ISCT Head Office
Canada

Elena Cooper, MSc
ISCT ESP Committee
United States

Maryam Pasdar, BSc
ISCT ESP Committee
United States
Being recognized as a leader in driving efforts in workforce development, ISCT was invited to take part in the workshop on Training the Regenerative Medicine Workforce for the Future hosted by the Forum on Regenerative Medicine at the National Academies of Sciences, Engineering and Medicine (NASEM).
The virtual workshop, Co-Chaired by ISCT North America Regional VP, Patrick Hanley, featured sessions from ISCT Head Office Senior Manager Meagan Pasternak and ISCT ESP Members Elana Cooper and Maryam Pasdar, which addressed issues related to lack of workforce diversity and opportunities to support workforce development in the future.
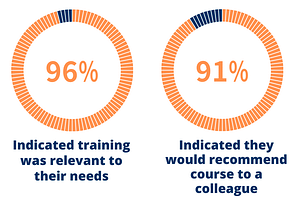
Identifying the limited number of skilled workers in manufacturing and product development as a major barrier to growth in the CGT sector, ISCT partnered with The National Science Foundation (NSF) Engineering Research Center for Cell Manufacturing Technologies (CMaT) to develop and deliver the first Workforce Development in Biomanufacturing course.
Developed by field experts from academic, regulatory, clinical, and commercial domains, the online program introduces key topics in cell and gene therapy manufacturing not only to up skill the existing biomanufacturing workforce but also provide those transitioning from other related fields with the fundamental knowledge and skillset unique to the space.
Following the successful global launch of the pilot program in June 2022, ISCT announced its plans to continue to offer the course on an annual basis and introduce three additional program dates throughout the year.
To further build on the learning of the online program, ISCT’s partnership with CMaT will see the introduction of the first innovative hands-on, on-site course, which will launch mid-2023.
Feedback from Inaugural Course

Queenie Jang,
BSc (Pharmacy), MBA
CEO, ISCT
Canada
“This biomanufacturing course, delivered in collaboration with CMaT, is a first step to address the broader needs of CGT training for both industry and academia. The overwhelmingly positive feedback we have received from the participants who took part in the course shows us that we are on the right track.”
“CMaT is tremendously excited to collaborate with ISCT and offer this critically needed course for the Cell Therapy Industry. We were humbled to see the community’s strong support and enthusiasm for this first offering”

Krishnendu Roy, PhD
Robert A. Milton Chair, CMaT
United States
“Working in the GMP facilities alongside experienced teachers and other international students enabled viable discussions, effective sharing of experiences and allowed us to put theory into practice.”

Katja Sirviö
MSc (Pharm)
Kuopio Center for
Gene and Cell Therapy
Finland

Alejandro Barquero
MPharm, MSc
Advent Bioservices Ltd
United Kingdom
“If you want to broaden your network and have fruitful exchanges with other field professionals from different ATMP backgrounds and countries, this is definitely the program for you.”
In partnership with ANd&AT and Universidad de Granada, supported by an educational grant from Miltenyi, ISCT was delighted to launch the first ISCT Master Scholarship Program in 2021/2022.
Available to ISCT members in Europe, the program offers three full-tuition scholarships to enroll in the Master Degree or Specialization Degree in Manufacturing of Advanced Therapy Medicinal Products, offered by the University of Granada/ Andalusian Network for the design and translation of Advanced Therapies (ANd&tAT).
The first of its kind, these programs are uniquely designed for technologies presently working in Good Manufacturing Practice (GMP)-compliant facilities within Europe producing cell therapy, gene therapy or tissue-engineered products for human use.
Following the demand for the 2021/2022 course, ISCT will continue to offer these scholarships on a biennial basis.
Congratulations to the awardees of the inaugural ISCT Europe Master Programme Scholarship on their completion of the 2021/2022 Master in Manufacturing of ATMPs at the University of Granada/ Andalusian Network for the design and translation of Advanced Therapies.
2021/2022 Scholars:
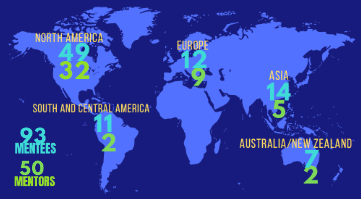
In 2022, ISCT was delighted to continue The ESP Mentoring Program. Designed to help guide the career and professional development of early-stage CGT professionals, the program connects ESPs with ISCT’s global network of CGT experts.
Since its launch in 2018, the mentoring program has seen significant growth with benefits for both mentee and mentor applications alike. 2022 was a stand out year for the program with 93 participating mentees and 50 participating mentors from across the globe.

Lindsay Davies, PhD
CellTherEx Consulting
Sweeden
“I have been a mentor for the last 2 years and really enjoyed speaking with ESPs, learning where they feel they need support and help for further development and tailoring sessions and topics to their individual requirements. I believe I have learnt as much from them as they have been able to learn from me.”
“If I were to describe in one word my early experience with my mentor, this word would be EMPOWERING! It’s exactly what I was hoping for. Early on they helped me identify my professional strengths and deficits, and helped me expand my research ideas.”

Diana Cirstea, MD
Massachusetts
General Hospital
United States
“I have had the opportunity to work with big names in the field, learn from them and apply the knowledge in my developing research. Since committee members are from around the world, I have gained exposure to global perspectives and updates on the latest methods and study directions“
Reza Yarani, PhD
Stanford University
United States
The ISCT ESP Leadership Development Program offers Early Stage Professionals (ESPs) a chance to join an ISCT Committee in a formal ESP position for a 2-year term providing a practical and immersive learning experience.
In 2022, ISCT was delighted to receive 46 applications from ESP members interested in the program. These applications were from over 18 countries globally, and positions were awarded on participating scientific, industry, regulatory, regional and communications committees.
Through the program, ESPs receive guidance from key global opinion leaders while collaborating on real-world projects in their area of interest that will advance the field of cell and gene therapy.
With a focus on expanding access to educational resources on a global level, ISCT leaders were motivated to develop and deliver a strong portfolio of webinars featuring both member-exclusive and open-access content. Many of the webinars were delivered as curated series leveraging ISCT expert committees to engage with international experts to share perspectives and knowledge with the wider community.
To foster global engagement and learning, ISCT encouraged cross-collaboration between regional committees to host joint webinar series, bringing together the ISCT North America and the ISCT South & Central America regional executive committees to deliver a series on Cancer Vaccines for Oncological Therapy and Implementing a new clinical CAR T program: Manufacturing your own.
2022 marked an unprecedented level of engagement for the ISCT webinar series, with members accessing content live and on demand.
ISCT has a specialized career site focused on CGT talent and opportunities. In 2022, the site saw record-breaking engagement. with the increasing demand for talent, ISCT is proud to provide a resource tool that presents quality opportunities to talent, ensuring that career seekers are able to use it as a reliable and selective point of access for first-choice job options in cell and gene therapy.
The career centre also provides job posters access to a select group of global CGT professionals with the expertise and skills needed to thrive in a fast-growing field to help streamline the recruitment process.

ISCT develops and delivers key expertise in regulatory and quality operations through the collaborations of several key stakeholder committees.
These include the Global Regulatory Task Force, which assesses and actions opportunities for regulatory harmonization and consultations, the regional legal and regulatory affairs committees, which closely track and advise on decisions from regional regulatory bodies, and the Lab Practices Committee, which focuses on the development of key member-facing resources that bolster the knowledge available to ISCT members.
Since 2004, ISCT has been the host coordinating over 15 stakeholder organizations in the annual FDA Cell Therapy Liaison Meeting (CTLM). These closed meetings enable the cell and gene therapy community to inform the FDA of specific concerns, challenges and recent developments to advance the regulatory field.
Each year, the CTLM report provides valuable coverage of key topics for regulatory consideration, summarizing the presentations that took place at the meeting, and providing key takeaways that are particularly useful to sector stakeholders.
The 2022 FDA CTLM focused on the following topics:
ISCT offers regulatory review and consultation from cell and gene therapy experts for regulatory authorities worldwide.
Review and recommendations are made by our Global Regulatory Task Force, as well as by regional Legal and Regulatory Affairs Committees in our North America, Europe, and Australia and New Zealand regions. Each consultation consists of significant comments, suggested revisions, and edits for regulatory documentation.
Below are the major guidance reviews in 2021:



Regulatory Consultations and Supports
Consultation with WHO: WHO Considerations for Regulatory Convergence of Cell and Gene Therapy Products (February 7 -9)
ISCT was invited by WHO to participate in a closed 3 day consultation meeting, following formal comments submitted in January 2022 on global convergence among health authorities. The meeting objectives were to review a draft paper, discuss key issues identified from public consultation, reach consensus and propose improvements. The meeting was attended by ISCT representatives Karen Nichols, Esq. ISCT Chief Regulatory Officer and Gabrielle O’Sullivan PhD, Australia and New Zealand LRA Co-Chair.
Meeting with Representatives of the Pharmaceutical Industry on the Hospital Exemption (November 23, 2022)
ISCT was invited to participate in a closed meeting with relevant organizations and subject matter experts to discuss implications for the pharma industry of the existence of exemption pathways for the manufacture of ATMPs, views of the pharma industry regarding the different implementation framework of Hospital Exemption in various member states in the EU. The meeting was attended by ISCT representatives Miguel Forte, MD, PhD ISCT President-Elect and Christopher Bravery, PhD, ISCT EU LRA Chair

Queenie Jang, BSc (Pharmacy), MBA
Chief Executive Officer
Canada
ISCT has made significant headway in offering leading educational materials for stakeholders, through renewed partnerships across the globe. From translational research to good manufacturing practices and regulatory understanding, ISCT members now have more access than ever to the key knowledge and networks that they need to become an effective part of the fast-expanding CGT workforce.
ISCT has focused its investment in education towards manufacturing this year, to address a critical demand for expertise in the global market. By expanding pathways for professionals to become key players in the sector, ISCT is opening doors for newcomers to the field.
Our Society continues to make major impacts. Throughout 2021, we have made strategic investments in several key resources, including our redesigned website and member library, and the development of educational programs targeting areas of critical importance in the CGT workforce. More than ever, our focus is on cultivating the emerging generation of cell and gene therapy professionals, promoting high standards at a global level, and providing the platform for cutting-edge knowledge and practices to flow through the sector.
Alongside this, our community continues to show its vibrant spirit. In the past year, we have seen the first iteration of a highly successful ISCT TV program. We’ve also seen the emergence of a truly community-driven member forum, and thought-provoking discussions amongst our global membership. Underlying all of this was a highly successful ISCT 2021 New Orleans VIRTUAL Annual Meeting. Each of these initiatives plays a crucial role in keeping connections in the field ready and fresh despite the great challenges of the ongoing pandemic.
ISCT 2021 met with great success, despite the screen fatigue that has characterized much of the last year and a half as we continue to face the effects of the COVID-19 pandemic. Under the guidance of a strong organizing committee, we delivered a dynamic and LIVE-focused program to our global membership. The virtual program for this meeting has become a permanent resource for ISCT members across the sector.
Despite the difficulties of having to connect virtually, ISCT members and leaders have truly brought the globe together in advancing cell and gene therapies in this past year.
From the work of past ISCT president Massimo Dominici in kickstarting a strategic partnership with the WHO to develop the first harmonized CGT nomenclature, to work of our MSC committee in solidifying terminology, our Society is having a breakthrough year in the regulatory space.
We continue to collaborate with key sector partners like ICCBBA, while contributing guidances and contributions to regulatory agencies in the EU and Australia especially. Our collaborations with the FDA, meanwhile, led to a landmark webinar discussing the implications of the end of the Enforcement Discretion Period, a key discussion that has great value for stakeholders across the sector globally.
Alongside all of this, ISCT is working hard to address an urgent global need: the rising demand for cell and gene therapy professionals. Working with our strategic partners, we have created programs to help open doors for newcomers to the field. Beginning with the Andalusian Network for the Development and Translation of Advanced Therapies (ANd&tAT), we have created a flexible, digital program aimed at teaching best practices and principles for those working in cell manufacturing, especially in Europe. With our partners at CMaT, we are now developing a key program that aims to bridge a critical gap in the number of skilled personnel across companies and clinical manufacturing sectors in particular.
Workforce development is the greatest challenge that lies ahead for cell and gene therapies to come to full fruition. Our field is driven by the passion of a diverse and relatively small group of pioneers in regulatory, commercial, and research enterprises. To advance further, we must harness this passion to develop paths and open doors for both interested professionals and fresh newcomers alike. If this year has proven anything, it’s that ISCT members are ready to take on this critical challenge.
This year we saw, time and time again, ISCT members coming together to collaborate and advance our field
As a truly global organization, ISCT leverages a regional model to deploy solutions to global issues on a regional level. Spearheaded by passionate volunteer leadership, who understand the needs and concerns unique to each region, ISCT addresses the emergent needs of CGT professionals across the globe. With partnerships including regional partner organizations, regulatory bodies, and stakeholder communities, each of the ISCT regional committees drives the advancement of translational science at a high global standard.
Our committees work autonomously to deliver key scientific meetings, stimulate membership growth, and develop regional professional networks. Through 2022, our committees worked hard to develop the groundwork for new committees with a special focus on early-stage professionals and facilitated several major events that have continued to build consensus across the sector.
In 2022, to further the society’s commitment to workforce development, ISCT formed three new regional early-stage professionals subcommittees in Asia, Australia and New Zealand and South-Central America. Supported by the respective regional executive committee, these subcommittees were created to promote regional and global engagement and networking for early-stage professionals providing them with resources and career guidance to ensure their success.
Championed by regional volunteers, the Asia, Australia and New Zealand and South-Central America subcommittees are in full swing developing key initiatives to drive ESP membership and engagement in each region. ISCT is currently working on establishing ESP subcommittees in both North America and Europe for 2023.
ISCT regional committees deliver high-quality programmes globally, with autonomous planning supported by the society’s global network and resources. As restrictions eased post-pandemic, regional executive committees were excited to return to in-person meetings and reconnect face-to-face with the wider ISCT community.
With a reignited vigour to connect cell and gene therapy professionals globally, 2022 was a particularly active year for regional events. ISCT regional committees partnered and collaborated with several external groups and organizations as well connecting with other ISCT committees.
In August, the Australia & New Zealand Regional Executive Committee hosted its first in-person regional meeting post-pandemic. The highly anticipated event welcomed 137 delegates to Brisbane, Australia, and featured workshops and panel discussions with particular attention to quality, regulatory affairs, education, training, commercialization and clinical practice.
Meanwhile, in North America, the leadership team hosted a virtual interactive member-focused regional town hall meeting in October to connect and engage with members in the region. The meeting facilitated open discussions focused on workforce development and retention challenges, including both commercial and academic perspectives.
To facilitate cross-regional collaboration, The North America and South & Central America Regional Executive Committees partnered to present a two-part webinar series. The first installment discussed the clinical manufacturing of CAR T Cells, and the second explored the mechanism, safety and efficacy of mRNA-based cancer vaccines for oncological therapy. The collaborative series brought together subject matter experts and key opinion leaders from across both regions and received over 550 registrants from across the globe.


In Australia & New Zealand, ISCT leaders held a joint virtual scientific meeting with the Biotherapeutics Association of Australasia (BAA) to address developments in cell and gene therapies. The two-day event, held in February 2022, hosted scientific presentations featuring both international and regional speakers to discuss the latest developments in the clinical and commercial delivery of cell and gene therapies and the latest regulatory updates relevant to the region.
Leaders in Asia were delighted to continue its partnership with the Korean Society of Blood and Marrow Transplantation (KSBMT) with a joint session on gene editing and gene therapy at the International Congress of Blood and Marrow Transplantation (ICBMT), the 27th Annual congress of KSBMT in September. The long-standing partnership between ISCT and KSBMT is a key partnership for the region. It fosters international cooperation in education and research in the field of hematopoietic stem cell transplantation and cell & gene therapy.


In May, the ISCT Asia Regional Executive Committee partnered with the Japanese Society for Transplantation and Cellular Therapy (JSTCT 2022) to host a joint session on ‘Strategies to Improve Classical CBT by Introducing New Cellular Therapies’. The session was chaired by Satoshi Takahashi, MD, PhD, Past ISCT Asia Regional Vice-President, JSTCT 2022 President and included a remote presentation from William YK Hwang, MBBS, MMed, FRCP, FAMS, ISCT Asia Regional Vice-President Elect.
In October, the South and Central America Executive Committee again partnered with the Brazilian Association of Cellular and Gene Therapy (ABTCel-Gen) for the XII Congress of ABTCel – Gen in Rio de Janeiro. ISCT invited speakers participated both in-person and virtually, leading discussions on topics such as manuscript writing, cell therapy and therapies for cancer through sessions and interactive workshops. Alongside event participation, The South and Central America regional leaders were proud to sponsor the top-scoring abstract awards and provide a special abstract supplement showcasing all accepted abstracts from the meeting publication in Cytotherapy, the official journal of ISCT.




Janet Macpherson, PhD
Australia

C. Russell Y. Cruz, MD, PhD
USA
Available to ISCT members, Telegraft is the go-to resource to keep up to date with the latest Society activities and important developments in cell and gene therapy.
2022 brought exciting updates for Telegraft, with the launch of the ‘Telegraft Hub,’ the new online home for ISCT news and community. Alongside monthly e-newsletters, ISCT members now have access to the online interactive hub, allowing them to connect with peers and access key updates and curated content.
The Telegraft Editorial Board works to engage and connect the whole ISCT community across academia, science, regulation and commercial by sharing insightful discussions and up-to-date information on critical issues affecting the field of cellular therapy.

Michele Sugrue, MT
USA

Wouter Van’t Hof, PhD
USA

Joaquim Vives, PhD
Portugal

Rounak Dubey, MBBS, MD
India

Shyam Bhakta, MD, MBA
India

Ashley Krull, BSc, PhD
USA

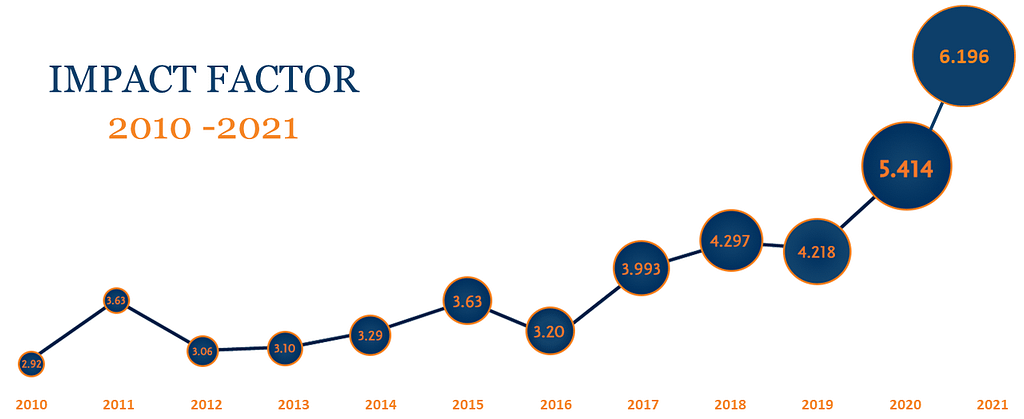
Cytotherapy®, the official journal for the International Society for Cell and Gene Therapy (ISCT), publishes novel and innovative results from high quality scientific and clinical studies in the fields of cell and gene therapy. In 2021, Cytotherapy has continued to successfully advance and expand the field of cell and gene therapy for clinical researchers, oncologists, hematologists, physicians, and regulatory experts alike. The Journal had a 6.196 impact factor for 2022, a new record from which the editorial board hopes to grow further.
You can read issues of Cytotherapy® here.
Follow Cytotherapy® on Twitter to stay up to date on all the latest news and publications.
In January 2022, Dr. Rachel Burga was appointed as the new assistant editor for Cytotherapy. With a background in biomedical engineering and immunology, Rachel is the Principal Scientist of Cell Therapy at Obsidian Therapeutics. She has been an active member of ISCT since 2016 and currently sits on the Early-Stage Professionals Committee as Co-Chair.
As Assistant Editor, Rachel will work closely with Senior Editor Dr. Donald G. Phinney and Commissioning Editor, Dr. Patrick Hanley, to raise awareness of the Cytotherapy brand. In her first term, Rached helped recruit outstanding content for the journal and work closely with the ISCT membership to ensure that the journal continues to support the Society’s mission.

Rachel Burga, PhD
Assistant Editor, Cytotherapy
United States
Each year, ISCT hosts the Insta-Your-Cells Photo Challenge, calling on community members to submit interesting images from under the microscope. These images are selected to feature on the front cover of Cytotherapy®, the Official Journal of ISCT, showcasing the fascinating world of cells to peers around the globe.
The winning image of the 3rd annual Insta Your Cells Photo Challenge was submitted by Philippe Cohen, MD, PhD, France. ISCT Head Office prepared a framed keepsake to celebrate this iconic first-place image.
Take a look at the stunning submissions selected to be featured on the front cover of Cytotherapy® in 2022.


Emily Hopewell, PhD
Indiana University School Of Medicine
United States

Patrick Hanley, PhD
Children’s National Hospital
United States
Recognizing the current skills gaps in CGT translation as a critical issue for the sector, in November 2022, ISCT formed the ISCT Workforce Development Committee.
Led by Co-chairs Emily Hopewell, PhD and Patrick Hanley, PhD the committee will provide strategic guidance to identify, develop and deliver ISCT training and development programs.
Programs will focus on the specific needs of professions based on their region, industry, and skill level to support the sustainable growth of the cell and gene therapy sector.
Throughout 2023, the committee will focus on identifying and prioritizing urgent needs for training and educational programs for professionals working across the full spectrum of translational science, starting from pre-clinical development through to patient access, to develop and deliver comprehensive programs to upskill and train the existing CGT workforce.


Laertis Ikonomou, PhD
The State University of New York at Buffalo
United States
In October 2021, the ISCT Presidential Task Force on the Use of Unproven and/or Unethical Cell and Gene Therapies formally became the ISCT Committee on the Ethics of Cell and Gene Therapy (ECGT). This change is intended to reflect the committees revised mission to identify key ethical issues associated with the development, regulatory authorization, and distribution of cell and gene therapies.
In 2022, the Committee re-affirmed its position on, and concerns with, speculative immune cell banking. Following the 2022 US federal court ruling in favor of California Stem Cell Treatment Center, Inc., and Cell Surgical Network Corporation, the Committee contributed to publishing an ISCT position and press release, highlighting the dangers of unproven cell therapies to patient safety.
Undertaking its broader remit, the ECGT Committee developed and published informed positions providing guidance and clarity on complicated ethical matters within the CGT space. The Committee has undertaken the task of developing resources to address ethical topics, and potential issues, arising from the EU Hospital Exemption and the US expanded access pathway. The Committee organized a roundtable session at the ISCT 2022 San Francisco Annual Meeting addressing both pathways, and published a position paper on Hospital Exemption within the framework of safety and ethical interests of patients. On January 19, 2022, the Committee launched the ISCT Expanded Access Working Group.
Over the course of 2022, the Committee has worked to develop a guide for the general public, which analyses the regenerative medicine industry, focusing on distinctive features of unproven cell and cell-based products, suggesting practical strategies to address marketing of such products, and providing an overview of reporting mechanisms available to patients across different jurisdictions globally. This comprehensive guide will be published in 2023 and provides an important resource to protect patients globally.

Patricia J. Zettler, JD
The Ohio State University
United States

The ISCT Expanded Access Working Group was launched with the mandate to identify and address practical, ethical, and regulatory issues that arise from the use, and potential misuse, of the expanded access pathway by various CGT stakeholders.
Since its launch, the Working Group has been developing informed perspectives on expanded access to generate educational resources that promote innovation in translational research while upholding the highest ethical standards. The Working Group submitted a short report reviewing the history and current state of the US expanded access pathway, and highlighting three emerging areas of concern. The report will be published in Cytotherapy in early 2023.
The overarching objective of the Working Group is to further refine its position on ethical issues arising from the use, and potential misuse, of the expanded access pathway, culminating in the development of a code of ethics. In developing this code of ethics, ISCT will seek out input and endorsement from other organizations in the field.
ISCT acknowledges the service of its members, volunteering expertise, time, and energy to the vast task of advancing the field of cell and gene therapies.
From mentoring the next generation to publishing key standards and fostering relationships across the sector, ISCT members set the gold standard.
We acknowledge these Society leaders who have completed terms of service in a formal position in 2021.
Your hard work and dedication have continually pushed the society to new heights, and we cannot wait to connect again soon:

John Rasko, AO, MBBS, PhD, FRCPA, FRACP, FAHMS
Chair, Strategic Advisory Council (Immediate Past President)
June 2020 – June 2022
Royal Prince Alfred Hospital
Sydney, Australia

Lizette Caballero, BS, MT(ASCP)
Global Secretary
June 2019 – June 2022
UCSF Blood and Marrow Transplant Lab
San Francisco, CA, United States

Rajiv Khanna, PhD AO
Australia & New Zealand, Regional Vice-President
June 2020 – June 2022
QIMR Berghofer Medical Research Institute
Brisbane, Australia

Mitchell Cairo, MD
North America, Regional Vice-President
June 2020 – June 2022
New York Medical College
New York, NY, United States

Karen Nichols, Esq.
Chief Regulatory Officer
March 2016 – June 2022
Vertex Pharmaceuticals
Cambridge, MA, United States

Vicki Antonenas, BSc, MSc
Elected Member Technologist
June 2020 – June 2022
Sydney Cellular Therapies Laboratory,
Westmead Hospital
Australia

Patrick Hanley, PhD
Co-Chair Immuno-Gene
Therapy Committee
Children’s National Hospital
United States

Giuseppe Orlando, MD, PhD
Co-Chair Gastrointestinal Committee
Wake Forest University
United States

Rachele Ciccocioppo, MD
Co-Chair Gastrointestinal Committee
University of Verona
Italy

George Muschler, MD
Co-Chair Orthopedic & Musculoskeletal Therapies
Cleveland Clinic
United States

Christian Jorgensen, MD, PhD
Co-Chair Orthopedic & Musculoskeletal Therapies
University Hospital Lapeyronie
France

Sarah Chan, Ma PhD
ECGT Committee
University of Edinburgh
United Kingdom

Andrew Hoffman, DVM, DVSc
Exosomes Committee
University of Pennsylvania
United States

John Fink, MBA
Co-Chair Process Development and Manufacturing Committee
PerkinElmer, Inc.
United States

Emily Hopewell, PhD
Indiana University School of Medicine
United States

Aaron Tze Kai Tan
Stanford University
United States

Lizbeth Diaz Polo, MD
University of San Martin de Porres
Spain

Jordan Greenberg, PhD
Shoreline Biosciences
United States

Shabnum Patel, PhD
CARGO Therapeutics
United States

Nancy H. Collins, PhD
University of Toledo
Toledo, OH, United States

Satyam Arora, MD
Super Speciality Paediatric Hospital and Post Graduate Teaching Institute
India

Lab Practices Committee
Vicki Antonenas, BSc, MSc
Westmead Hospital
Australia

Europe Legal & Regulatory Affairs Committee
Paula Salmikangas, PhD
NDA Group
Finland

Cytotherapy®, the official journal for the International Society for Cell and Gene Therapy (ISCT), publishes novel and innovative results from high quality scientific and clinical studies in the fields of cell and gene therapy.In 2021, Cytotherapy has continued to successfully advance and expand the field of cell and gene therapy for clinical researchers, oncologists, hematologists, physicians, and regulatory experts alike. The Journal had a 5.414 impact factor for 2020, a new record from which the editorial board hopes to grow further.
This year, ABTCel-Gen, a CGT-focused society based in South & Central America, joined as an affiliate society to Cytotherapy. This landmark partnership creates a strong pathway to publication for affiliate members, while drawing from a proven pool of translational expertise.
Take a look at our Talking with Giants series, launched in 2019, which showcases leaders in the field of cell and gene therapy through informal question and answer articles. Talking with Giants is featured at the start of each issue of Cytotherapy.
You can read issues of Cytotherapy® here.
This year, Cytotherapy® also launched a Twitter account to share fresh updates and resources.
Each year, ISCT hosts the Insta-Your-Cells Photo Challenge, calling on community members to submit interesting images from under the microscope. These images are selected to feature on the front cover of Cytotherapy®, the Official Journal of ISCT, showcasing the fascinating world of cells to peers around the globe.
This year, ISCT Head Office also prepared a framed keepsake to celebrate an iconic first place image, submitted by Pradyut Paul, PhD, University of Wisconsin-Madison, United States.

Take a look at the stunning submissions selected to be featured on the front cover of Cytotherapy® this year.



Janet Macpherson, PhD
Australia

C. Russell Y. Cruz, MD, PhD
USA
Available to ISCT members, Telegraft is the go-to resource to keep up to date with the latest Society activities and important developments in cell and gene therapy.
2022 brought exciting updates for Telegraft, with the launch of the ‘Telegraft Hub,’ the new online home for ISCT news and community. Alongside monthly e-newsletters, ISCT members now have access to the online interactive hub, allowing them to connect with peers and access key updates and curated content.
The Telegraft Editorial Board works to engage and connect the whole ISCT community across academia, science, regulation and commercial by sharing insightful discussions and up-to-date information on critical issues affecting the field of cellular therapy.

Nancy H. Collins, PhD
USA

Satyam Arora, MD
USA

Rounak Dubey, MBBS, MD
India

Shyam Bhakta, MD, MBA
India

Ashley Krull, BSc, PhD
USA

Michele Sugrue, MT
USA

Wouter Van’t Hof, PhD
USA

Joaquim Vives, PhD
Portugal
ISCT acknowledges the service of its members, volunteering expertise, time, and energy to the vast task of advancing the field of cell and gene therapies.
From mentoring the next generation to publishing key standards and fostering relationships across the sector, ISCT members set the gold standard.
We acknowledge these Society leaders who have completed terms of service in a formal position in 2021.
Your hard work and dedication have continually pushed the society to new heights, and we cannot wait to connect again soon:

John Rasko, AO, MBBS, PhD, FRCPA, FRACP, FAHMS
Chair, Strategic Advisory Council (Immediate Past President)
June 2020 – June 2022
Royal Prince Alfred Hospital
Sydney, Australia

Lizette Caballero, BS, MT(ASCP)
Global Secretary
June 2019 – June 2022
UCSF Blood and Marrow Transplant Lab
San Francisco, CA, United States

Rajiv Khanna, PhD AO
Australia & New Zealand, Regional Vice-President
June 2020 – June 2022
QIMR Berghofer Medical Research Institute
Brisbane, Australia

Mitchell Cairo, MD
North America, Regional Vice-President
June 2020 – June 2022
New York Medical College
New York, NY, United States

Karen Nichols, Esq.
Chief Regulatory Officer
March 2016 – June 2022
Vertex Pharmaceuticals
Cambridge, MA, United States

Vicki Antonenas, BSc, MSc
Elected Member Technologist
June 2020 – June 2022
Sydney Cellular Therapies Laboratory,
Westmead Hospital
Australia

Patrick Hanley, PhD
Co-Chair Immuno-Gene
Therapy Committee
Children’s National Hospital
United States

Giuseppe Orlando, MD, PhD
Co-Chair Gastrointestinal Committee
Wake Forest University
United States

Rachele Ciccocioppo, MD
Co-Chair Gastrointestinal Committee
University of Verona
Italy

George Muschler, MD
Co-Chair Orthopedic & Musculoskeletal Therapies
Cleveland Clinic
United States

Christian Jorgensen, MD, PhD
Co-Chair Orthopedic & Musculoskeletal Therapies
University Hospital Lapeyronie
France

Sarah Chan, Ma PhD
ECGT Committee
University of Edinburgh
United Kingdom

Andrew Hoffman, DVM, DVSc
Exosomes Committee
University of Pennsylvania
United States

John Fink, MBA
Co-Chair Process Development and Manufacturing Committee
PerkinElmer, Inc.
United States

Emily Hopewell, PhD
Indiana University School of Medicine
United States

Aaron Tze Kai Tan
Stanford University
United States

Lizbeth Diaz Polo, MD
University of San Martin de Porres
Spain

Jordan Greenberg, PhD
Shoreline Biosciences
United States

Shabnum Patel, PhD
CARGO Therapeutics
United States

Nancy H. Collins, PhD
University of Toledo
Toledo, OH, United States

Satyam Arora, MD
Super Speciality Paediatric Hospital and Post Graduate Teaching Institute
India

Lab Practices Committee
Vicki Antonenas, BSc, MSc
Westmead Hospital
Australia

Europe Legal & Regulatory Affairs Committee
Paula Salmikangas, PhD
NDA Group
Finland
As a truly global organization, ISCT leverages a regional model to deploy solutions to global issues on a regional level. Spearheaded by passionate volunteer leadership, who understand the needs and concerns unique to each region, ISCT addresses the emergent needs of CGT professionals across the globe. With partnerships including regional partner organizations, regulatory bodies, and stakeholder communities, each of the ISCT regional committees drives the advancement of translational science at a high global standard.
Our committees work autonomously to deliver key scientific meetings, stimulate membership growth, and develop regional professional networks. Through 2022, our committees worked hard to develop the groundwork for new committees with a special focus on early-stage professionals and facilitated several major events that have continued to build consensus across the sector.
In 2022, to further the society’s commitment to workforce development, ISCT formed three new regional early-stage professionals subcommittees in Asia, Australia and New Zealand and South-Central America. Supported by the respective regional executive committee, these subcommittees were created to promote regional and global engagement and networking for early-stage professionals providing them with resources and career guidance to ensure their success.
Championed by regional volunteers, the Asia, Australia and New Zealand and South-Central America subcommittees are in full swing developing key initiatives to drive ESP membership and engagement in each region. ISCT is currently working on establishing ESP subcommittees in both North America and Europe for 2023.
ISCT regional committees deliver high-quality programmes globally, with autonomous planning supported by the society’s global network and resources. . As restrictions eased post-pandemic, regional executive committees were excited to return to in-person meetings and reconnect face-to-face with the wider ISCT community.
With a reignited vigour to connect cell and gene therapy professionals globally, 2022 was a particularly active year for regional events. ISCT regional committees partnered and collaborated with several external groups and organizations as well connecting with other ISCT committees.
In August, the Australia & New Zealand Regional Executive Committee hosted its first in-person regional meeting post-pandemic. The highly anticipated event welcomed 137 delegates to Brisbane, Australia, and featured workshops and panel discussions with particular attention to quality, regulatory affairs, education, training, commercialization and clinical practice.
Meanwhile, in North America, the leadership team hosted a virtual interactive member-focused regional town hall meeting in October to connect and engage with members in the region. The meeting facilitated open discussions focused on workforce development and retention challenges, including both commercial and academic perspectives.
To facilitate cross-regional collaboration, The North America and South & Central America Regional Executive Committees partnered to present a two-part webinar series. The first installment discussed the clinical manufacturing of CAR T Cells, and the second explored the mechanism, safety and efficacy of mRNA-based cancer vaccines for oncological therapy. The collaborative series brought together subject matter experts and key opinion leaders from across both regions and received over 550 registrants from across the globe.


In Australia & New Zealand, ISCT leaders held a joint virtual scientific meeting with the Biotherapeutics Association of Australasia (BAA) to address developments in cell and gene therapies. The two-day event, held in February 2022, hosted scientific presentations featuring both international and regional speakers to discuss the latest developments in the clinical and commercial delivery of cell and gene therapies and the latest regulatory updates relevant to the region.
Leaders in Asia were delighted to continue its partnership with the Korean Society of Blood and Marrow Transplantation (KSBMT) with a joint session on gene editing and gene therapy at the International Congress of Blood and Marrow Transplantation (ICBMT), the 27th Annual congress of KSBMT in September. The long-standing partnership between ISCT and KSBMT is a key partnership for the region. It fosters international cooperation in education and research in the field of hematopoietic stem cell transplantation and cell & gene therapy.


In May, the ISCT Asia Regional Executive Committee partnered with the Japanese Society for Transplantation and Cellular Therapy (JSTCT 2022) to host a joint session on ‘Strategies to Improve Classical CBT by Introducing New Cellular Therapies’. The session was chaired by Satoshi Takahashi, MD, PhD, Past ISCT Asia Regional Vice-President, JSTCT 2022 President and included a remote presentation from William YK Hwang, MBBS, MMed, FRCP, FAMS, ISCT Asia Regional Vice-President Elect.
In October, the South and Central America Executive Committee again partnered with the Brazilian Association of Cellular and Gene Therapy (ABTCel-Gen) for the XII Congress of ABTCel – Gen in Rio de Janeiro. ISCT invited speakers participated both in-person and virtually, leading discussions on topics such as manuscript writing, cell therapy and therapies for cancer through sessions and interactive workshops. Alongside event participation, The South and Central America regional leaders were proud to sponsor the top-scoring abstract awards and provide a special abstract supplement showcasing all accepted abstracts from the meeting publication in Cytotherapy, the official journal of ISCT.

Building Consensus and Community
Motivated to stay true to the essence of ISCT as a truly global translation-focused community of experts, ISCT made major strategic progress in 2022, with significant investments in community development, workforce development and addressing ethical issues. Working in collaboration with ISCT leadership, the Society enhanced the value of its membership value across all disciplines for leaders and newcomers alike as well as developed key resources and drove consensus to combat the critical issues facing the wider CGT sector. As the ISCT community continues to grow, the Society is perfectly positioned to empower the field to drive the translation of research into safe, effective and accessible treatments with long-lasting impact.

Excited to welcome delegates to the first in-person ISCT meeting post Pandemic, ISCT was determined to make a significant impact with ISCT 2022 San Francisco. Welcoming 1,646 delegates from across the globe, ISCT 2022 was the largest ISCT Annual Meeting to date and was proud to serve as a key event reconnecting the wider CGT community. A standout feature of the meeting was the redesign of the ISCT Scientific program, a strategic initiative inspired to better cater to the interests of all delegates, across the full range of CGT development and deployment.
The Translational Pathway Program, reflects ISCT’s role in driving the translation of scientific research into safe and effective therapies that can be deployed to patients. The new program was designed to mirror the therapeutic development process and integrated perspectives across the CGT sector to address key topics at all stages of translation.
As a counter-part to the Translational Pathway Program, ISCT also launched the Roundtable Program at ISCT 2022. Unique in the cell and gene therapy sector, this program provides a platform for delegates to debate and collaboratively develop consensus and solutions to practical problems and barriers in the field. Recognizing the breadth and depth in expertise of Annual Meeting delegates, ISCT developed this unique platform to connect professionals from across the globe who share the same goals and encounter the same difficulties.
“ISCT broadened its focus, through a whole series of roundtables, to cover wider challenges experienced across the industry, from generating return on investment to managing the supply chain. ISCT will continue to monitor the efficacy of solutions generated at the meeting and continue to work with all stakeholders to ensure an increasing number of patients are able to benefit from cell and gene therapies.”

Bruce Levine, PhD
University Of Pennsylvania
United States
The rapid growth of the sector has introduced new ethical concerns associated with the development, regulatory authorization, and distribution of cell and gene therapies. Recognizing this, ISCT has worked to develop internal infrastructure so that the Society remains a leading voice on critical and topical ethical issues.
Undertaking a broader remit to identify these key ethical issues, ISCT has introduced the Committee on the Ethics of Cell and Gene Therapy (ECGT). Formally the ISCT Presidential Task Force on the Use of Unproven and/or Unethical Cell and Gene Therapies, this new multidisciplinary committee regroups key stakeholder perspectives to promote the ethical development of the sector.
To further refine the Society’s position on ethical issues, ISCT formed the ISCT Expanded Access Working Group. The overarching objective of the Working Group is to further refine its position on ethical issues arising from the use, and potential misuse, of the expanded access pathway, culminating in the development of a code of ethics.
Under the guidance of the new ECGT committee, ISCT is now better positioned to give informed consensus on ethical issues. In 2022, following the 2022 US federal court ruling in favor of California Stem Cell Treatment Center, Inc., and Cell Surgical Network Corporation, ISCT published a press release highlighting the dangers of unproven cell therapies to patient safety.
With Industry members representing a vital part of ISCT DNA, in 2022, ISCT worked to adapt the industry program to better meet the needs of our growing community. In collaboration with the ISCT Industry leadership, ISCT worked to evaluate how to adapt our program gaining feedback from our members at the forefront of driving the translation of cell and gene therapies.
At its core, ISCT Industry Membership provides the ability to network, discuss current challenges in Cell and Gene Therapy and examine potential solutions with global experts from a variety of disciplines. As part of the restructure, ISCT has adapted the membership program to two levels, Patron and Associate, both of which offer access to networking events, monthly strategic discussion forums and the opportunity to contribute to the development of sector advancing educational resources.
The newly enhanced program also welcomed The Asia Pacific Regional Commercialization Committee, created to foster strong industry relationships in the region.
The launch of the new membership program marks a significant milestone for ISCT, with a record number of new members joining in 2022. ISCT Industry leadership looks forward to continuing to grow our global community and support those working to expand the development and delivery of safe and effective cell and gene therapies for patients.

Anthony Ting, PhD
Bone Therapeutics
United States
ISCT continues to advance scientific translation through the expansion and improvement of its peer-reviewed scientific journal, Cytotherapy. Under the close collaboration of its editorial board, with support from ISCT Head Office and our publishers at Elsevier, the Journal has attained a record-breaking impact factor at 6.196.
In 2022, Cytotherapy published 132 articles including, reviews, short repots, correspondence and editorials. The Journal received a record number of downloads and citations solidifying its position as the first choice in cell and gene therapy translation publishing.

Donald Phinney, PhD
Senior Editor
United States

Patrick Hanley, PhD
Commissioning Editor
United States
Take a look at the stunning submissions selected to be featured on the front cover of Cytotherapy® in 2022.
Building Consensus and Community
Motivated to stay true to the essence of ISCT as a truly global translation-focused community of experts, ISCT made major strategic progress in 2022, with significant investments in community development, workforce development and addressing ethical issues. Working in collaboration with ISCT leadership, the Society enhanced the value of its membership value across all disciplines for leaders and newcomers alike as well as developed key resources and drove consensus to combat the critical issues facing the wider CGT sector. As the ISCT community continues to grow, the Society is perfectly positioned to empower the field to drive the translation of research into safe, effective and accessible treatments with long-lasting impact.

Excited to welcome delegates to the first in-person ISCT meeting post Pandemic, ISCT was determined to make a significant impact with ISCT 2022 San Francisco. Welcoming 1,646 delegates from across the globe, ISCT 2022 was the largest ISCT Annual Meeting to date and was proud to serve as a key event reconnecting the wider CGT community. A standout feature of the meeting was the redesign of the ISCT Scientific program, a strategic initiative inspired to better cater to the interests of all delegates, across the full range of CGT development and deployment.
The Translational Pathway Program, reflects ISCT’s role in driving the translation of scientific research into safe and effective therapies that can be deployed to patients. The new program was designed to mirror the therapeutic development process and integrated perspectives across the CGT sector to address key topics at all stages of translation.
As a counter-part to the Translational Pathway Program, ISCT also launched the Roundtable Program at ISCT 2022. Unique in the cell and gene therapy sector, this program provides a platform for delegates to debate and collaboratively develop consensus and solutions to practical problems and barriers in the field. Recognizing the breadth and depth in expertise of Annual Meeting delegates, ISCT developed this unique platform to connect professionals from across the globe who share the same goals and encounter the same difficulties.
“ISCT broadened its focus, through a whole series of roundtables, to cover wider challenges experienced across the industry, from generating return on investment to managing the supply chain. ISCT will continue to monitor the efficacy of solutions generated at the meeting and continue to work with all stakeholders to ensure an increasing number of patients are able to benefit from cell and gene therapies.”

Bruce Levine, PhD
University Of Pennsylvania
United States
The rapid growth of the sector has introduced new ethical concerns associated with the development, regulatory authorization, and distribution of cell and gene therapies. Recognizing this, ISCT has worked to develop internal infrastructure so that the Society remains a leading voice on critical and topical ethical issues.
Undertaking a broader remit to identify these key ethical issues, ISCT has introduced the Committee on the Ethics of Cell and Gene Therapy (ECGT). Formally the ISCT Presidential Task Force on the Use of Unproven and/or Unethical Cell and Gene Therapies, this new multidisciplinary committee regroups key stakeholder perspectives to promote the ethical development of the sector.
To further refine the Society’s position on ethical issues, ISCT formed the ISCT Expanded Access Working Group. The overarching objective of the Working Group is to further refine its position on ethical issues arising from the use, and potential misuse, of the expanded access pathway, culminating in the development of a code of ethics.
Under the guidance of the new ECGT committee, ISCT is now better positioned to give informed consensus on ethical issues. In 2022, following the 2022 US federal court ruling in favor of California Stem Cell Treatment Center, Inc., and Cell Surgical Network Corporation, ISCT published a press release highlighting the dangers of unproven cell therapies to patient safety.
With Industry members representing a vital part of ISCT DNA, in 2022, ISCT worked to adapt the industry membership program to better meet the needs of our growing community. In collaboration with ISCT Industry leadership, ISCT worked to tailor its Committees using feedback from our members to create a program that supports those who are at the forefront of driving the translation of cell and gene therapies.
At its core, ISCT Industry Membership provides the ability to network, discuss current challenges in Cell and Gene Therapy and examine potential solutions with global experts from a variety of disciplines. As part of the restructure, ISCT has adapted the membership program to two levels, Patron and Associate, both of which offer access to monthly strategic discussion forums, networking events, and the opportunity to contribute to the development of sector advancing educational resources.
The newly enhanced program also welcomed The Asia Pacific Regional Commercialization Committee, created to foster strong industry relationships in the region.
The launch of the new membership program marks a significant milestone for ISCT, with a record number of new members joining in 2022. ISCT Industry leadership looks forward to continuing to grow the ISCT global industry community and support those working to expand the development and delivery of safe and effective cell and gene therapies for patients.

Anthony Ting, PhD
BRL C> Consulting
United States

ISCT continues to advance scientific translation through the expansion and improvement of its peer-reviewed scientific journal, Cytotherapy. Under the close collaboration of its editorial board, with support from ISCT Head Office and our publishers at Elsevier, the Journal has attained a record-breaking impact factor at 6.196.
In 2022, Cytotherapy published 132 articles including, reviews, short repots, correspondence and editorials. The Journal received a record number of downloads and citations solidifying its position as the first choice in cell and gene therapy translation publishing.

Donald Phinney, PhD
Senior Editor
United States

Patrick Hanley, PhD
Commissioning Editor
United States

Take a look at the stunning submissions selected to be featured on the front cover of Cytotherapy® in 2022.

Bruce Levine, PhD
President
June 2020 – June 2022
Barbara and Edward Netter Professor in Cancer Gene Therapy
University of Pennsylvania
Philadelphia, PA, United States

Jacques Galipeau, MD
President-Elect
June 2020 – June 2022
University of Wisconsin-Madison
Madison, WI, United States

John Rasko, AO, MBBS, PhD, FRCPA, FRACP, FAHMS
Chair, Strategic Advisory Council (Immediate Past President)
June 2020 – June 2022
Royal Prince Alfred Hospital
Sydney, Australia

Lizette Caballero, BS, MT(ASCP)
Global Secretary
June 2019 – June 2022
UCSF Blood and Marrow Transplant Lab
San Francisco, CA, United States

Emily Hopewell, PhD
Global Treasurer
June 2020 – June 2023
Indiana University School of Medicine
Zionsville, Indiana

Bryan Choi, PhD
Asia, Regional Vice-President
June 2021 – June 2023
Strategic Center For Regenerative Medicine (SCRM)
Inha University
Incheon, Korea


Massimiliano Gnecchi, MD, PhD
Europe, Regional Vice-President
June 2021 – June 2023
University Of Pavia & IRCCS Policlinico San Matteo Hospital
Pavia, Italy

Mitchell Cairo, MD
North America, Regional Vice-President
June 2020 – June 2022
New York Medical College
New York, NY, United States

Antonio Carlos Campos de Carvalho, MD, PhDSouth and Central America, Regional Vice-President
June 2021 – June 2023
Institute Of Biophysics
Federal University Of Rio De Janeiro
Rio De Janeiro, Brazil

Daniel J. Weiss, MD, PhD
Chief Scientific Officer
June 2016 – June 2022
University of Vermont
Burlington, VT, United States

Anthony Ting, PhD
Chief Commercialization Officer
May 2020 – May 2022
Athersys
Cleveland, OH, United States

Karen Nichols, Esq.
Chief Regulatory Officer
March 2016 – June 2022
Vertex Pharmaceuticals
Cambridge, MA, United States

Dominique Farge, MD, PhD
Elected Member MD
June 2021 – June 2023
Greater Paris University Hospitals – AP-HP
Paris, France

Sowmya Viswanathan, PhD
Elected Member PhD
June 2020 – June 2022
University Health Network and University of Toronto
Canada

Vicki Antonenas, BSc, MSc
Elected Member Technologist
June 2020 – June 2022
Sydney Cellular Therapies Laboratory,
Westmead Hospital
Australia

Diane Kadidlo, BSc, MT(ASCP), SBB
Elected Member Technologist
June 2021 – June 2023
Molecular And Cellular Therapeutics
University Of Minnesota
St Paul, Minnesota, United States

Donald Phinney, PhD
Senior Editor of the Journal
October 2018 – Present
Professor at The Scripps Research Institute
Jupiter, FL, United States

Queenie Jang, BSc (Pharmacy), MBA
Chief Executive Officer
ISCT
Vancouver, BC, Canada



Kevin Bosse, PhD RAC
Nationwide
Children’s Hospital
United States
Rachel Burga, PhD
Obsidian Therapeutics
United States
The ISCT Early Stage Professionals Committee (ESP) strives to engage and connect ESP with the wider CGT sector on a regional and global level. Co-Chaired by Kevin Bosse, PhD RAC and Rachel Burga, PhD, the committee provides networking opportunities and career development resources for professionals to excel in their careers.
The committee hosts a variety of tailored events and workshops, such as the ISCT ESP Mentoring Program and the ISCT ESP Leadership Development program helping connect professionals with key opinion leaders and subject matter experts.
In 2022, ISCT formed three regional ESP subcommittees in Asia, Australia and New Zealand and South Central America to drive ESP membership and increase interactions between ESPs and regional leadership.