If you are seeing this, please disable your adblock software to view the full version of our annual report!
Our Community Re-Imagined
ISCT President Bruce Levine shares a Virtual Happy Hour with the ISCT Head Office. June 2020
“Tortilla chip, anyone?”

John Rasko, AO, MBBS, PhD,
FRCPA, FRACP, FAHMS
Outgoing ISCT President
(May 2018- May 2020)
Australia
We are the Consensus Builders
I am proud of where we stand today, as consensus builders in a rapidly-growing sector that has, against the odds, found itself invigorated by the fight against the COVID-19 pandemic.
The annual report serves as a moment to reflect upon how much we have achieved in pursuit of our mandate, to review where we must go, and to renew our dedication to the job that remains to be done.
In 2020, ISCT made tough calls and followed through on its commitments, by rallying together as a cohesive community. Bringing key voices together despite the ongoing economic and public health challenges of the ongoing COVID-19 pandemic, ISCT has solidified its position as a consensus builder in the field of cell and gene therapy.
This is reflected across the cell and gene therapy translational spectrum. Two examples serve as highpoints: the Chief Scientific Officer’s spotlight during our ISCT 2020 Paris Virtual Annual Meeting, which advanced scientific understanding by bringing leaders in the field to conduct a timely and urgent discussion on critical studies; and the annual FDA Cell Therapy Liaison Meeting, which brings industry voices into conversation with a key regulatory body. Another relevant example lies in the consortium of sector stakeholders that ISCT brought together to conduct productive discussions with Google on its policies around the advertisement and information on both emerging and unproven therapies.
There are many challenges that remain ahead of us, but if this difficult time has proven anything, it is that ISCT has the spirit, the vision, and the members needed to meet them. Regardless of where we are, we have always earned our moniker of a “truly global” society, staying connected and engaging with our shared mission to better human healthcare across any distance across the globe.
Transitions provoke occasions for reflection on achievements and on future opportunities for success.
As one of the first academic societies to commit to a fully virtual conference in 2020, the speed with which we were able to pivot was nothing short of miraculous. It usually takes well-over a year to organize our face-to-face annual meetings and ISCT owes the greatest debt to our meeting co-chairs, Organizing Committee, and head office for pulling it off in just two months so triumphantly! If COVID-19 has brought any good to scientific meetings, it is the introduction of improved access for those who might be unable to travel for any reason. I have little doubt that in the long term, future meetings post-vaccine will benefit from hybrid features which include the opportunity for simultaneous streaming and physical attendance.
When I was elected to the post of President of ISCT, I set out a plan to bring ISCT initiatives to a new level: improving the scope and quality of our communications, enhancing gender equity and mentorship, strengthening our capacity to lead the sector through strategic liaisons and partnerships, re-energising the Presidential Task Force on the Use of Unproven and/or Unethical Cell and Gene Therapies and expanding our membership numbers. Above all, I wanted communication – both inward and outward – to be a feature of my Presidency. We are in a position to act as a powerful and influential voice for the field, and to advance the mission of bringing cell and gene therapies to fruition.
My two-year presidency at ISCT comes to a close.
As I pass the torch to the capable hands of my trusted friend and colleague, Dr. Bruce Levine, I am struck by how our mission has become increasingly visible in the pursuit of better global healthcare outcomes. Cell and gene therapies are taking a position of prominence to make a real impact in the lives of patients around the globe, and it is happening before our eyes. What a thrill to be part of history here and now.
Alongside Dr. Levine, several ISCT members have stepped up to the mantle of leadership on our Board of Directors. I would like to welcome Jacques Galipeau, Rajiv Khanna, Mitchell Cairo, Anthony Ting, Sowmya Viswanathan, and Vicki Antonenas to this esteemed group. There are many others who have also come to lead our scientific, regulatory, and industry communities, and you will see them recognized throughout this report.
I am continually inspired, and genuinely excited to see where we can go next in my role as chair of the ISCT Strategic Advisory Council. 2021 marks the start of a new decade and a new chapter for both our Society and our field.
As a proud Australian, I have been truly honoured and humbled to be the first President of ISCT from the southern hemisphere, specifically the Asia-Pacific region. As we begin to twist the curve of the pandemic, our wide ISCT community should look forward to brighter days ahead with confidence. We will re-connect in person, and I for one cannot wait.
Thank you for being part of the ISCT family, to the long-haulers and to those joining us this year.
May each of you, and your communities, stay safe as we get ever closer to a renewed normal.
See you soon, mate!

Bruce Levine, PhD
ISCT President (2020 – 2022)
United States
An Inspiring and Resilient Community
In a year marked deeply by the ongoing COVID-19 pandemic, our professionals drive a concerted push that grows stronger than ever as many cell and gene therapies advance from discovery to development to commercialization.
2020 marked significant challenges, and yet, there is great opportunity ahead. We are at the cusp of a new wave in cell and gene therapies, and I know that this Society will be on the leading edge.
This year challenged us individually and as a community. We adapted to come together virtually, and we supported one another through continued commitments and community connection. At the start of the pandemic, we started the #ISCTTogether campaign, with our Telegraft newsletter and on social media. We highlighted the COVID-19 research and expertise of our members, and we shared stories and learnings of operating in the new and challenging environment.
You might know that we saw a record-breaking mentorship kick-off, successful webinars leveraging an industry-leading platform, committee calls converted to video calls, and global connectivity on multiple platforms. These are the results of a resilient community, trained to expect the unexpected, coming together and adapting.
Made more challenging by pandemic disruptions, the need to share perspectives, provide mentoring and support, and work together across boundaries of distance, backgrounds, and perspectives is crucial. One of my first goals as President of ISCT has been to conduct a ‘world tour’.
Bruce Levine, ISCT President World Tour: Talking with the ISCT South and Central America Regional Executive Committee
I was honored to have this chance to sit down and work with each of the ISCT Regional Committees and our Scientific Chairs and Global Regulatory Committees to learn about what lies ahead around the globe, and where I could contribute. Along with attending meetings of the Commercialization Committee, these experiences have driven home what it means for this society to be multi-disciplinary and truly global.
Alongside the drive to connect, ISCT is standing up for our core values. We must advocate for inclusion and diversity, fairness in opportunity, and fairness in access that characterize our approach to the challenge of advancing cell and gene therapy translation. In the months since I have taken up the President’s gavel, we have laid out a formal statement of values. These values help to define our collective commitment to the integrity and trust that have fueled this Society since its inception and speak to the spirit of our collaboration.
To get to where we now are, it has taken massive effort and vision beyond the horizon. I thank and commend John, my predecessor, in his role as President, for helping to steer the Society to where it stands today. I would also acknowledge those who have completed their terms within the Board of Directors. To Catherine Bollard, Ngaire Elwood, Lynn O’Donnell, Miguel Forte, Nadim Mahmud, and Jeannette Bloom – thank you for your dedication and service to the Society.
“Building trust… connections… collaborations… and the path forward – this is what ISCT is about.”

Queenie Jang, BSc (Pharmacy), MBA,
Chief Executive Officer
Canada
Re-imagining the Community in an Unprecedented Year
2020 was an unprecedented year with unprecedented challenges for our Society. In response to the COVID-19 pandemic, ISCT rapidly repositioned to meet the changed needs of a global membership largely embedded in the fight on the frontlines.
By taking action decisively and collaboratively, this Society proved that it has what it takes to lead the field in meeting emergent and systemic challenges. From the pivot from ISCT 2020 Paris to Paris Virtual, to the expanding scope of our collaborations across the sector, to the additions we have introduced to our committees and leadership rosters, ISCT has made extraordinary impacts in a year that was unrelenting in its challenges.
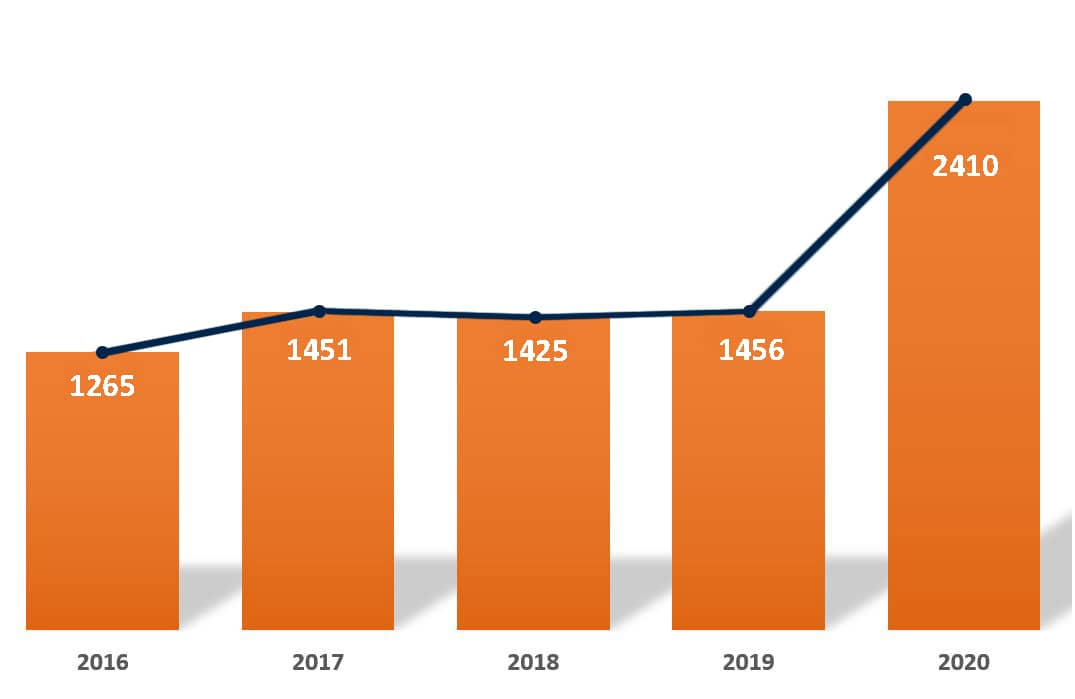
“In 2020, our society gained more than a thousand new members.”
It has been an important year for our mission. Alongside the challenges we have faced, cell and gene therapies have come to the forefront of public interest. As many of us know, the sector is forecasted to almost double in size within the next few years. Similarly, this community is growing at a rapid pace.
While we could not connect in person, ISCT was able to come together to bring people and organizations, ideas and resources to address some of the key issues facing the sector today. This was no more apparent than during the ISCT 2020 Paris Virtual Annual Meeting.
With little more than eight weeks between public health decisions and our original meeting planned for a large scale in-person gathering in Paris, we devoted immense effort from our speakers, exhibitors, organizing committee, and head office, to pivot to a safe, virtual meeting model. We managed to develop and deliver a robust scientific program, key spotlight presentations honing on urgent topics, and a venue for emerging science to find fresh and interested eyes.
Along the way, we saw the community spirit that enrichens every ISCT Annual Meeting express itself in style. In the virtual environment, we wanted to deliver an experience that brought a sense of ‘coming home’, and of escaping, if only for a few hours, the day-to-day experience of the COVID-19 pandemic. We brought in avatars; speaker panels that were not just colleagues – but friends; networking, and as much of the in-person experience as we could translate to virtual.
We got to see great moments like the Paris Virtual Avatar Challenge (Thanks to Maroun Khoury). There were heartwarming social media posts of attendees embracing the Paris theme of our meeting, and finding fun alongside the hard work and innovation it takes to bring science to fruition.
We have grown stronger as a global community
In a year where many of our members fought on the frontlines of the COVID-19 pandemic, we saw an unprecedented increase in mentorship and giving back. Despite the pandemic’s impact on innovation – delaying clinical trials, disrupting supply chains, making in-person networking impossible – ISCT members found the means…
…to support one another, to endure, and to stay engaged during this year.
Our Board of Directors
The ISCT Board of Directors consists of:
- President (Chair of the Board of Directors)
- President-Elect
- Immediate Past President (Chair of the Strategic Advisory Council)
- Global Secretary
- Global Treasurer
- Regional Vice Presidents
- Chief Scientific Officer
- Chief Regulatory Officer
- Chief Commercialization Officer
- 4 Elected ISCT Members
- Senior Editor of the Society’s Journal
- Chief Executive Officer.
The Board of Directors is the main administrative body which manages the governance and strategic oversight of the Society.
- ISCT 2019-2020 Board of Directors
- ISCT 2020-2021 Board of Directors
Chair

John Rasko, AO, MBBS, PhD, FRCPA, FRACP, FAHMS
President
June 2018 – June 2020
Royal Prince Alfred Hospital
Sydney, Australia
Directors

Bruce Levine, PhD
President-Elect
June 2018 – June 2020
Barbara and Edward Netter Professor in Cancer Gene Therapy
University of Pennsylvania
Philadelphia, PA, United States

Catherine Bollard, MBChB, MD
Chair, Strategic Advisory Council (Immediate Past President)
June 2018 – June 2020
Children’s National Health System
George Washington University
Washington, DC, United States

Lizette Caballero, BS, MT(ASCP)
Global Secretary
June 2019 – June 2022
UCSF Blood and Marrow Transplant Lab
San Francisco, CA, United States

Emily Hopewell, PhD
Interim Global Treasurer
June 2019 – June 2020
Indiana University School of Medicine
Zionsville, Indiana

Oscar Lee, MD, PhD
Asia, Regional Vice-President
June 2019 – June 2021
National Yang- Ming University
Taiwan

Ngaire Elwood, PhD
Australia & New Zealand, Regional Vice-President
June 2018 – June 2020
Murdoch Children’s Research Institute
Melbourne, Australia

Joan Garcia-Lopez, MD, PhD
Europe, Regional Vice-President
June 2019 – June 2021
Director of Research and Education Banc de Sang i Teixits Barcelona, Spain

Lynn O’Donnell, PhD
North America, Regional Vice-President
June 2018 – June 2020
Ohio State University, James Cancer Hospital
Columbus, OH, United States

Patricia Rocco, MD, PhD
South and Central America, Regional Vice-President
June 2019 – June 2021
Federal University of Rio de Janeiro
Rio de Janeiro, Brazil

Daniel J. Weiss, MD, PhD
Chief Scientific Officer
June 2016 – June 2022
University of Vermont
Burlington, VT, United States

Miguel Forte, MD, PhD
Chief Commercialization Officer
June 2016 – June 2020
Bone Therapeutics
Brussels, Belgium

Karen Nichols, Esq.
Chief Regulatory Officer
March 2016 – June 2021
Vertex Pharmaceuticals
Cambridge, MA, United States

Elizabeth Stenger, MD, MSc
Elected Member MD
June 2019 – June 2021
UPMC Children’s Hospital of Pittsburgh
Pittsburgh, PA, United States

Nadim Mahmud, MBBS, PhD
Elected Member PhD
June 2018 – June 2020
University of Illinois College of Medicine
Chicago, IL, United States

Jeannette Bloom, MBA, MT(ASCP)SBB
Elected Member Technologist
June 2018 – June 2020
Baylor College of Medicine
Houston, TX, United States

Anne Lamontagne, MSc
Elected Member Technologist
June 2019 – June 2021
Clinical Cell and Vaccine Production Facility
University of Pennsylvania
Philadelphia, PA, United States

Donald Phinney, PhD
Senior Editor of the Journal
October 2018 – Present
Professor at The Scripps Research Institute
Jupiter, FL, United States

Queenie Jang, BSc (Pharmacy), MBA
Chief Executive Officer
ISCT
Vancouver, BC, Canada
Chair

Bruce Levine, PhD
President
June 2020 – June 2022
Barbara and Edward Netter Professor in Cancer Gene Therapy
University of Pennsylvania
Philadelphia, PA, United States
Directors

Jacques Galipeau, MD
President-Elect
June 2020 – June 2022
University of Wisconsin-Madison
Madison, WI, United States

John Rasko, AO, MBBS, PhD, FRCPA, FRACP, FAHMS
Chair, Strategic Advisory Council (Immediate Past President)
June 2020 – June 2022
Royal Prince Alfred Hospital
Sydney, Australia

Lizette Caballero, BS, MT(ASCP)
Global Secretary
June 2019 – June 2022
UCSF Blood and Marrow Transplant Lab
San Francisco, CA, United States

Emily Hopewell, PhD
Global Treasurer
June 2020 – June 2023
Indiana University School of Medicine
Zionsville, Indiana

Oscar Lee, MD, PhD
Asia, Regional Vice-President
June 2019 – June 2021
National Yang- Ming University
Taiwan

Brisbane, Australia

Joan Garcia-Lopez, MD, PhD
Europe, Regional Vice-President
June 2019 – June 2021
Director of Research and Education Banc de Sang i Teixits Barcelona, Spain

Mitchell Cairo, MD
North America, Regional Vice-President
June 2020 – June 2022
New York Medical College
New York, NY, United States

Patricia Rocco, MD, PhD
South and Central America, Regional Vice-President
June 2019 – June 2021
Federal University of Rio de Janeiro
Rio de Janeiro, Brazil

Daniel J. Weiss, MD, PhD
Chief Scientific Officer
June 2016 – June 2022
University of Vermont
Burlington, VT, United States

Anthony Ting, PhD
Chief Commercialization Officer
May 2020 – May 2022
Athersys
Cleveland, OH, United States

Karen Nichols, Esq.
Chief Regulatory Officer
March 2016 – June 2021
Vertex Pharmaceuticals
Cambridge, MA, United States

Elizabeth Stenger, MD, MSc
Elected Member MD
June 2019 – June 2021
UPMC Children’s Hospital of Pittsburgh
Pittsburgh, PA, United States

Sowmya Viswanathan, PhD
Elected Member PhD
June 2020 – June 2022
University Health Network and University of Toronto
Canada

Vicki Antonenas, BSc, MSc
Elected Member Technologist
June 2020 – June 2022
Sydney Cellular Therapies Laboratory,
Westmead Hospital
Australia

Anne Lamontagne, MSc
Elected Member Technologist
June 2019 – June 2021
Clinical Cell and Vaccine Production Facility
University of Pennsylvania
Philadelphia, PA, United States

Donald Phinney, PhD
Senior Editor of the Journal
October 2018 – Present
Professor at The Scripps Research Institute
Jupiter, FL, United States

Queenie Jang, BSc (Pharmacy), MBA
Chief Executive Officer
ISCT
Vancouver, BC, Canada
The ISCT Response to COVID-19
Pivoting to meet the Pandemic
The COVID-19 Pandemic raised significant challenges across the globe, and the cell and gene therapy sector was not immune. To meet the needs of our members in turbulent times, ISCT made several strategic pivots in 2020, emphasizing community, spotlighting discussions advancing COVID-19 treatments using cell and gene applications, and going virtual with the ISCT 2020 Annual Meeting.
Despite all of the challenges presented, ISCT membership grew to record numbers, emphasizing the need for community, connection and a common purpose. Despite the public health and economic impacts of the ongoing pandemic, ISCT continues to take the steps needed to bring cell and gene therapy stakeholders together to chart the path forward.
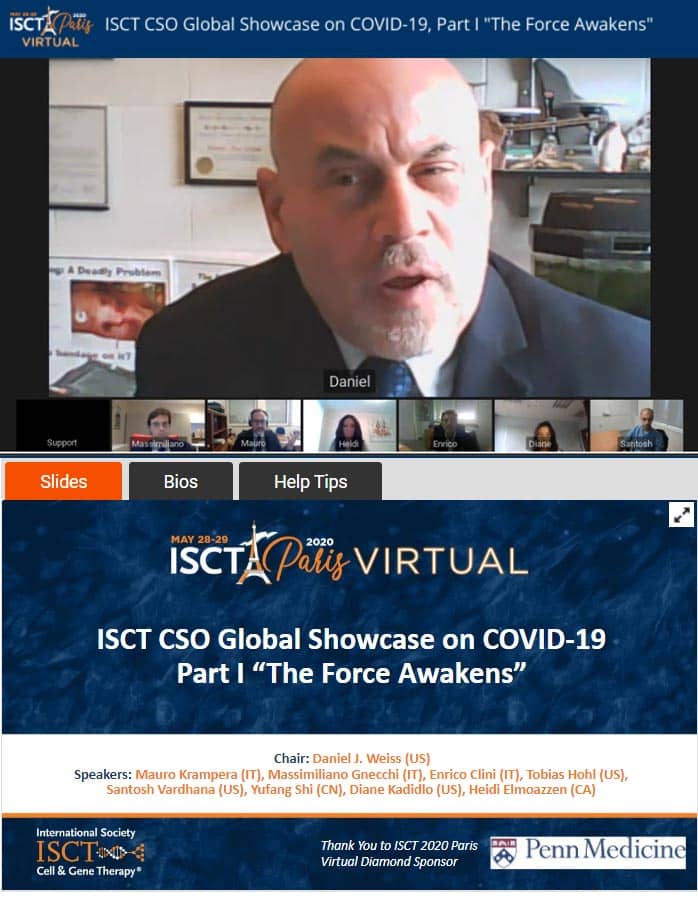
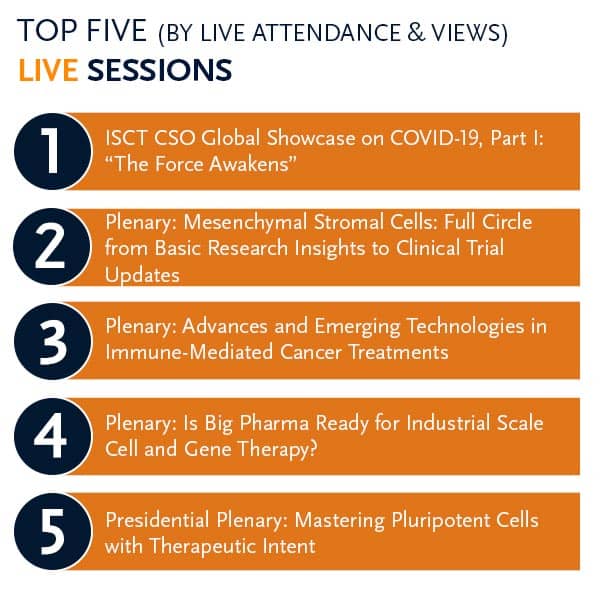
ISCT 2020 Chief Scientific Officer Spotlight on COVID-19
As part of the Society response to COVID-19, ISCT Chief Scientific Officer (CSO) Daniel J. Weiss led two sessions bookending the ISCT 2020 Paris Virtual Annual Meeting. This timely and critical pair of sessions provided key discussion examining the state of therapeutic efforts to address COVID-19, as well as the potential responses that could be leveraged by cell and gene therapy stakeholders.
Part I. “The Force Awakens”
This session focused on pathogenesis and on the experiences of health care workers on the front lines in Italy, New York City, and China. ISCT members shared their experiences at the forefront of both clinical care and mechanistic investigations, and the panel discussed the impact of travel and shipping restrictions on clinical cell therapy programs.
Part II. “A New Hope”
This session focused on novel cell-based and immunologic-based therapeutics. Facing an ongoing threat of emerging infectious disease, the panel considered what role cell therapies can play therapeutically and how studies should be conducted and interpreted. Through translational and clinical science, cell and gene therapies were discussed as a new hope for the development of novel therapeutics.
ISCT Showcase on COVID-19 Research
The COVID-19 pandemic put a spotlight on the possibilities that are already emerging in cell and gene therapy research. Through scientific presentations and the creation of a research spotlight, including several scientific review papers authored by ISCT members, ISCT has played a part in consolidating the scientific knowledge available on proven applications supported by research.
Simultaneously, through the actions of several key members and of the ISCT Presidential Task Force on Unproven/Unethical Cell and Gene Therapies (PTF), ISCT has played a key role in maintaining integrity, at a time when it is most needed, across many interactions between legitimate sector stakeholders and the public
Cell-based treatments for COVID-19
By Laertis Ikonomou, PhD
The COVID-19 pandemic has produced a rush of candidate treatments, currently evaluated worldwide for safety and efficacy. We discuss issues related to the development of cell-based interventions for COVID-19.
Current Status of Cell-Based Therapies for Respiratory Virus Infections: Applicability to COVID-19
By Maroun Khoury, Jimena Cuenca, Fernanda F. Cruz, Fernando E. Figueroa, Patricia R. M. Rocco, Daniel J. Weiss
Published in the European Respiratory Journal
Cell-Based Therapies for COVID-19: Proper Clinical Investigations are Essential
By Maroun Khoury, Patricia R.M. Rocco, Mauro Krampera, Ivan Martin, Sowmya Viswanathan, Donald G Phinney, Jan A. Nolta, Katarina LeBlanc, Jacques Galipeau and Daniel J. Weiss
Published in Cytotherapy
ISCT takes a stand against organizations marketing unproven COVID-19 cell and gene therapies
Wednesday, March 25, 2020
Following up on a statement made on March 20th, ISCT has issued a press release taking a stand against organizations marketing unproven COVID-19 cell and gene therapies.
ISCT 2020 Paris Virtual
ISCT CSO Global Showcase on COVID-19
Part I: “The Force Awakens”
- Review of Current Understanding of COVID-19 Pathogenesis
- Reports from the Front Lines
- Supply Chain Issues
LIVE at ISCT 2020 Paris Virtual
ISCT CSO Global Showcase on COVID-19
Part II: “A New Hope”
- MSC-based Approaches
- Immuno-Gene Therapy Approaches
Warnings on stem cells touted as COVID therapy
An Interview with ISCT Presidential Task Force Members Laertis Ikonomou, Daniel J. Weiss, and Megan Munsie by The Medical Republic regarding concerns about the marketing of unproven therapies during the pandemic.
ISCT CSO Daniel J. Weiss moderates Webinar on Potential COVID-19 Treatments Using Cell Therapies
Hosted by ARM, ARMF, and ISCT
Preying on Public Fears and Anxieties in a Pandemic: Businesses Selling Unproven and Unlicensed “Stem Cell Treatments” for COVID-19
By Leigh Turner, PhD
#ISCTTogether
Recognizing the need for community and connection, the ISCT community came together virtually to connect beyond the science to maintain resilience, develop hope, and work together in the face of the COVID-19 pandemic.
#ISCTTogether is a campaign of connection, envisioned by ISCT President Bruce Levine, to support one another through the critical challenges that continue to emerge as a result of the pandemic.
Showcasing stories of members adapting to new circumstances, learning to work together in a new normal, and developing connections that go beyond the science, #ISCTTogether speaks to the heart of the Society – inclusive collaboration in a truly global setting.
Pivoting to Virtual
For ISCT, the most visible and tangible impact of the pandemic was the difficult but necessary decision to pivot from the planned ISCT 2020 Annual Meeting in Paris, France, to a Virtual Meeting, with Paris recreated in the home offices and living rooms of ISCT members around the world.
While there were numerous challenges in pioneering a virtual event with less than 8 weeks to plan, the ISCT community came together and embraced the concept wholeheartedly. With over 2000 attendees, ISCT 2020 Paris Virtual broke new ground in encouraging truly global participation.
Living the Paris theme, the Organizing Committee worked to bring attendees together, recognizing that annual meetings serve not only as connective points for scientific collaboration, but also for an engaged community.
Presenters and attendees alike took the initiative several steps further, theming personal backdrops, bringing in Paris cheer, and most of all, having a moment of respite and good vibes that stood out among the days of a still-ongoing pandemic.
Embodying the spirit of ISCT fun, delegates took it upon themselves to insert their own photos into the ISCT 2020 Paris Virtual environment; the ISCT 2020 Paris Virtual Avatar Challenge was born!
Building Global Consensus in Cell and Gene Therapy
Moving the Field Forward
ISCT engages in unique collaboration between academia, regulatory bodies and industry partners in cell and gene therapy translation with a growing reputation as the community where these diverse partners can come together under the common mission of advancing CGT to improve patients’ lives. ISCT continues to build public trust and integrity for the field as a whole, as a powerful and visible voice for sector stakeholders.

In 2019, Google released an updated advertising policy, prohibiting advertisements for unproven or experimental medical techniques such as most stem cell therapy, cellular (non-stem) therapy and gene therapy, meant to limit the reach of bad actors that offer unproven, unlicensed and potentially harmful cell and gene based interventions. This action was lauded by many in the field, including ISCT, as a stand against unproven and unethical treatments.
Spearheaded by the ISCT Presidential Task Force on the Use of Unproven and/or Unethical Cell and Gene Therapies (PTF), ISCT brought together over 20 stakeholder organizations to consult with Google on unintended negative consequences on scientifically legitimate therapeutic uses of stem cells.
These supporting organizations represent an important collaborative effort to ensure high standards and public trust in advancing the development of cell and gene therapies, as proven products and ongoing clinical research become more prominent and require more direct public outreach.

CGT Standards and Harmonization



ISCT is composed of stakeholders and leaders across a translational spectrum, creating opportunities for productive collaborations that can lead to sector harmonization and standards that are accepted across commercial, academic, and regulatory domains.
ISCT contributed to consultations for several key agencies across the globe, including the International Standards Organization (ISO), where the ISCT Mesenchymal Stromal Cell Committee provided expert opinions on developing MSC definitions and standards; United States Pharmacopeia (USP), with which ISCT participates in developing new CGT relevant monographs; and the International Council for Commonality in Blood Banking Automation (ICCBBA), to standardize cell type/source abbreviations commonly used in ISBT 128 labeling standards.
ISCT is a community that connects the dots when it comes to developing the standards and harmonization needed to efficiently and effectively move the field forward.
FDA Cell Therapy Liaison Meeting (CTLM) 2020
ISCT continues to host an annual liaison meeting between key CGT sector stakeholders and the FDA Center for Biologics Evaluation and Research (CBER). Continuing to adapt to a changing world and re-imagining our programs, the 2020 CTLM was adapted to a virtual format, enabling expansion to the agenda and opening new possibilities for future meetings.
The 2020 CTLM addressed several critical topics including:
- Donor safety and standards
- Long-term tracing of cell therapy recipients
- Regulatory pathways for approved genetically modified products
- CMC comparability of CGT
- Framework for use of research grade materials in manufacturing of cellular therapies
Cytotherapy®
New URL. New Pipelines. Same Excellence.
//isct-cytotherapy.org
Cytotherapy®, the Official Journal of ISCT, publishes novel and innovative results from high quality scientific and clinical research in the fields of cell and gene therapy. ISCT continues to advance the field, and so the journal must advance as well. This is the journey of Cytotherapy building further upon its already significant roots, to create a consistent impact on the advancement of Cell and Gene therapies.
2020 marked significant shifts in the outreach and engagement of Cytotherapy. Building on the engagement of our scientific community within our annual meetings and in committees, the Journal continues to expand on its status as a visible and effective publication in the field where rigorous and exciting scientific advancement is discussed.
Our Editorial Leadership Team
“Patrick brings tremendous enthusiasm to the role of Commissioning Editor, which adds great energy to an already outstanding team of Editors. This enthusiasm has already translated into several exciting new initiatives at Cytotherapy, which I am confident will continue the journal’s rapid growth.”

Don Phinney, PhD
Senior Editor
United States

Patrick Hanley, PhD
Commissioning Editor
United States
“A key challenge is authenticity. We are all overwhelmed with emails every morning – ‘Dear Dr. Honorable Professor Hanley J Patrick, PhD, a fair greeting for the day. We invite you to publish a journal in the annals of unicorn surgery…’ It will be a challenge for us to eclipse the predatory journals and invite authors to contribute worthwhile content – but this is something we can achieve, by leveraging our extensive network and adding a personalized touch.”
(Hover or tap to remove the underlay)
A Warm Welcome to our New Editors
ISCT is pleased to welcome new Commissioning Editor for Cytotherapy, Dr. Patrick Hanley.
Within this role, Dr. Patrick Hanley joins the editorial board to develop the Journal’s selection of scientific publications, while liaising with key leaders in the field to develop focused and cutting-edge discourse. In this role, Dr. Hanley will help to identify areas of growth for the Journal to target, and set the groundwork for contributing authors and editorial board members to coordinate on emerging publications. He begins his role with the aim of expanding outreach to target a special issue of Cytotherapy for each year, in addition to an expansion of review and research articles.
Alongside this, Dr. Hanley will implement his vision on Journal development strategies in close partnership with Dr. Donald Phinney, Senior Editor of Cytotherapy, to steward the continued growth of the Journal going forward.
“I’d like to include Early Stage Professionals (ESPs) as much as possible. I think they (we?) are full of ideas, creativity, and enthusiasm. With proper mentorship they will be an invaluable resource for ISCT that will pay dividends now, but especially in the future…”
“…A part of that will be leaning on the scientific committees to include ESPs as appropriate and working with them to develop reviews, white papers, and special editions that are of interest to the community. “
Interested in learning more about what Dr. Hanley has envisioned?
Our New Associate Editors:
Also joining the Cytotherapy Editorial Board as associate editors in 2020 are Dr. Anna Pasetto and Dr. Qasim Rafiq, who will work under the leadership of Dr. Phinney to review submissions and plan the continued growth of the Journal’s impact.

Qasim A. Rafiq, PhD
University College London Department of Biochemical Engineering
United Kingdom
Dr. Rafiq completed his PhD at Loughborough University. He is now an Associate Professor of Cell and Gene Therapy Bioprocess Engineering at University College London and the Programme Director of an MSc programme focused on the Manufacture and Commercialisation of Stem Cell and Gene Therapies.

Anna Pasetto, PhD
Karolinska Institute
Department of Laboratory Medicine
Sweden
After completing her PhD at the Karolinska Institute, Dr. Pasetto trained with Dr. Steven A. Rosenberg at the National Cancer Institute, NIH, studying TCR genes and tumor infiltrating lymphocytes in metastatic solid cancers. She is now an Assistant Professor at the Karolinska Institute.
Special Thanks to Departing Associate Editors
We cannot move forward without recognizing the efforts of those who have brought us to where we are today. Dr. John Rasko and Dr. Massimo Dominici have stepped down from their roles as associate editors, after 11 years of service to the journal, and the Society.

John Rasko, AO, MBBS, PhD, FRCPA, FRACP, FAHMS
Australia

Massimo Dominici, MD
Italy
Thank you, John and Massimo, for your leadership.
As their roles come to a close, the Society and the Journal thank both Dr. Rasko and Dr. Dominici for their commitment and service to advancing the field through this important position, where their scientific and clinical experience provided key insights on decisions on many publications, helping to solidify the state of Cytotherapy as a high-impact, trusted journal within the field.
Inaugural Insta-Your-Cells Photo Challenge
An annual initiative introduced for 2019-2020, the Insta-Your-Cells photo challenge engages the cell therapy community and showcasing some of the fascinating imagery that can emerge from laboratory work. This year, over 50 images were submitted to compete for a featured spotlight on the front cover of Cytotherapy, and images garnering the most votes from ISCT members earned prizes from a pool including registration to ISCT 2020 Paris Virtual
Click the toggle below to see the images that were selected to be featured on the front cover of Cytotherapy in 2020
- Show all
- Hide All
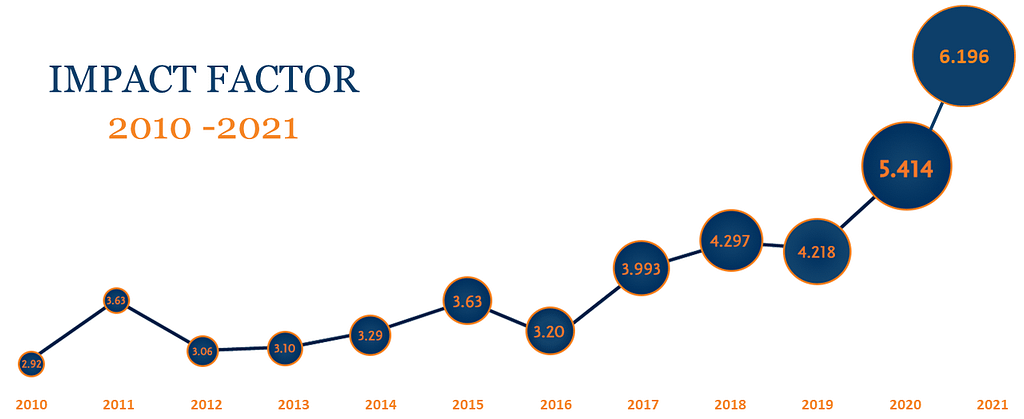
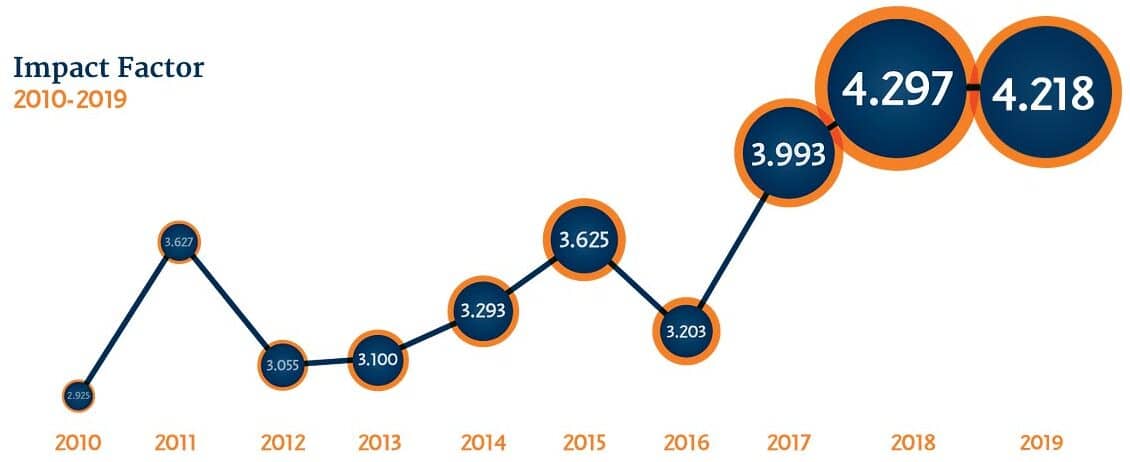
Making an Impact
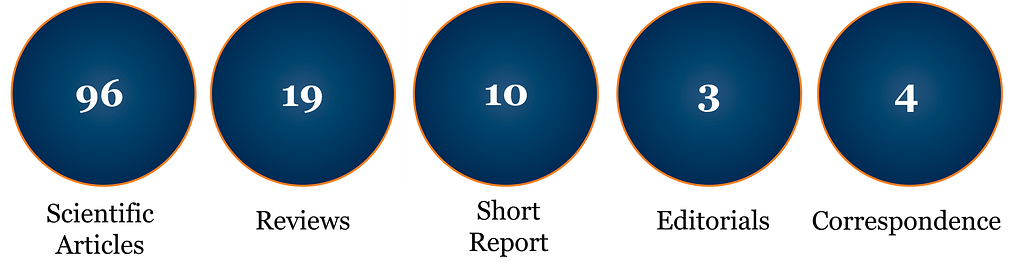
In 2020, Cytotherapy maintained a strong impact factor of 4.218, with a 43% increase in submissions, optimized editorial speeds, and strong quality control. A new focus within the scope of the Journal has been establishing a bank of open access content for key papers that are of critical interest to sector stakeholders. Open access publications now account for over 15% of Cytotherapy content.
The push towards becoming a more accessible publication has also driven the Editorial Board to provide a fresh and updated website, with new pipelines to publication connected to the ISCT Annual Meetings and other scientific events.
Talking with Giants Trilogy
This year, the ISCT Talking with Giants series featured a trilogy of interviews published in Cytotherapy to showcase some of the visionaries that spearheaded the breakthrough development of the first successful CAR T-Cell therapy, an inspiring milestone for cell and gene therapies. In this trilogy, learn about what kept Dr. Carl June motivated to keep driving pursuing the long road to a successful landmark therapy. Hear Dr. Bruce Levine‘s thoughts about the exciting trajectories ahead for cell and gene therapies. See the real impacts of this therapy through the story of Emily Whitehead, one of the first successful patients whose young life was saved by the treatment.
Excerpted from the Foreword by Dr. Donald Phinney, Senior Editor of Cytotherapy:
Alongside the International Society for Cell & Gene Therapy (ISCT), it is my privilege as Senior Editor of Cytotherapy to introduce to you this latest addition to the “Talking with Giants” series – a special “Talking with Giants Trilogy”…
Our trilogy begins with fellow ISCT member Dr. Carl June, MD, who almost needs no introduction. His pioneering work in T- cell immunotherapies led to the first successful CAR-T clinical trial in patients with refractory and relapsing chronic lymphocytic leukemia (CLL). Dr. June offers his insights into the rapid evolution of immunotherapy that has followed and reveals the motivation that drove him while working on this breakthrough trial…
Our Three Pillars


Rachele Ciccocioppo, MD
Deputy Chief Scientific Officer, ISCT
Italy
Cultivating a Community for Scientific Success
“I invite everybody interested in working with us to promote the cultural changes required for these emerging tools to find their rightful place within the therapeutic armamentarium, so they can progress to become a reality for patients”
To address the increasing need to augment our scientific voice, ISCT has introduced the role of Deputy Chief Scientific Officer (Deputy CSO). As the inaugural Deputy CSO, Dr. Rachele Ciccocioppo is tasked with the oversight of ISCT scientific committees, building internal consensus, standards, and positions that continue to advance ISCT scientific leadership.
“Undoubtedly, we are witnessing a tremendous expansion of the field of cell and gene therapies worldwide, and this represents an extraordinary opportunity for our patients,” says Rachele Ciccocioppo, ISCT Deputy Chief Scientific Officer, “As with all such opportunities, this brings positive and negative aspects that we must proactively manage.”
To Dr. Ciccocioppo, as more cell and gene therapies begin to enter a translational space, an increased need for professionals with roots in science, and with the skills needed for manufacturing and market implementation, is becoming apparent. “To ensure that ground-breaking scientific advances can be realized, it will be vital to cultivate a professional workforce, including bioengineers, health economists and bioinformatics. Any advancement in this area requires the collaboration of an expert network consisting of many kinds of scientists,” she says, identifying some of the key challenges facing the translation of CGT.
She continues, “…Another challenge is the harmonization of the regulatory framework, and the possibility to offer these kinds of therapies all around the world, while preventing exploitative gaps like medical tourism. The ability to effect harmonization of all these disparate but related disciplines is one of the exciting things about being part of ISCT.” As an experienced researcher herself, Dr. Ciccocioppo describes the importance of cooperation across regulators and researchers, pointing out that clear guidelines for market authorization and clinical trial research are a necessary early step that requires input from across a wide stakeholder community.
Drawing as well on her experience as a medical practitioner, Dr. Ciccocioppo concludes, “…Finally, a pillar of my mission will be to increase awareness of these therapies, which carry the advantage to perfectly fit within the needs of systems biology and precision medicine.” Dr. Ciccocioppo is a strong proponent of the use of cellular therapies for internal medicine, and has investigated the potential of Mesenchymal Stromal Cells as a treatment option for coeliac and inflammatory bowel diseases, as well as autoimmune enteropathy.

When asked how these goals can be achieved, she replies with enthusiasm, “All this work may be realized only within a strong, global community, like ours at ISCT, where I have enjoyed the privilege of sharing ideas and projects. So, I invite everybody interested to work with us to promote the cultural changes required for these emerging tools to find their rightful place within the therapeutic armamentarium, and progress to become a reality for patients!”

Jaap Jan Boelens, MD, PhD
Chair, Stem Cell Engineering Committee
USA
“Developments in this space are becoming more and more apparent in combinatorial settings, where the existing expertise within ISCT, combined with an increased presence of stem cell engineering therapies, will help us become a leader in this emerging science.”
The Stem Cell Engineering Committee is the newest ISCT Scientific Committee, created as a proactive initiative to advance the clinical translation of therapies involving stem cell engineering, leveraging existing ISCT expertise in hematopoietic stem cells (HSCs) as well as immunotherapy and gene therapies.
Under the leadership of founding chair Dr. Jaap Jan Boelens, this committee will establish and steward ISCT positions within the stem cell engineering space, with a focus on driving the clinical translation of engineered stem cells including HSCs. Additionally the committee will tackle the the integration of these engineering methods with novel immune and gene therapies.
Recognizing that developments in this space are emerging in parallel with new immune and gene therapies, this committee also looks forward to working collaboratively and closely with other ISCT scientific committees, beginning with the Immuno & Gene Therapy Committee, to explore the potential combinatorial applications of stem cell engineering therapies.

Ivan Martin, PhD
Chair, Mesenchymal Stromal Cell Committee
Switzerland
“In order to maintain strong leadership, it has become increasingly clear that it is important to tighten connections with both internal ISCT Committees and external international groups to support the challenges of developing and adopting MSC-based treatments.”
The translation of MSCs into clinical trials and products continues to develop on multiple fronts, with manifest interest by the scientific, technical, clinical, regulatory and commercial communities. The MSC Committee has consolidated its position as a vivid forum for discussion of upcoming events and topics in the MSC landscape, and has managed to communicate those internal reflections in the form of published statements or conference sessions.
In order to maintain strong leadership in this specialty, it has become increasingly clear that it is important to enlarge the forum beyond the borders of the Committee. The group has thus tightened connections with other ISCT Committees and opened to a dialogue with other international societies and working groups. We are convinced that this is the way to effectively serve the community in increasing awareness on the opportunities and challenges of developing and adopting MSC-based treatments.
2020 has highlighted key issues that remain to be addressed in the translation of MSC research to clinical treatments, including potency and manufacturing considerations. The ISCT MSC Committee continues to develop publications to address such issues, the most recent of which was published in March this year: “Improving mesenchymal stem/stromal cell potency and survival”, and continues to work with the International Standards Organization (ISO) on characterization and general guidelines for biobanking human MSCs

Sandeep Soni, MD
Co-Chair, Immuno & Gene Therapy Committee
USA
“Our committee brings relevant expertise and willing colleagues to identify critical challenges for the rapidly advancing field of immuno & gene therapies. I am excited to see us lead discussions spanning from the science to manufacturing, logistics, and regulation. “
Immunotherapy & gene therapies continue to be a core area for ongoing successful clinical translation and regulatory approval. From successful CAR-T therapies to leading research in NK-cells, to gene manipulation in both in-vivo and ex-vivo cells, ISCT leaders bring cutting-edge expertise in this specialty to advance scientific discourse. Near the onset of the COVID-19 pandemic, this committee was thus well placed to publish a comparative perspective focused on the viral symptoms of COVID-19 and symptoms targeted by ongoing immuno & gene therapeutic research.
In 2020, we welcome Dr. Sandeep Soni, who brings significant expertise as both an executive leader in industry, and as a researcher and clinician with a focus on ex-vivo gene editing, as co-chair of the Immuno & Gene Therapy Committee.

ISCT Committees represent an invaluable network of dedicated scientific leaders that work to develop and deliver papers that advance the field of cell and gene therapy. Below are a featured series of their publications throughout this year.
For more information on contributing to one of our committees, take a look at our Committee Directory.
Co-Chaired By:
| Sai-Kiang Lim, PhD A*STAR Institute of Medical Biology, Singapore | Bernd Giebel, PhD University of Duisberg-Essen, Germany |
1. International Society for Extracellular Vesicles and International Society for Cell and Gene Therapy statement on extracellular vesicles from mesenchymal stromal cells and other cells: considerations for potential therapeutic agents to suppress coronavirus disease-19
Co-Chaired By:
| Patrick Hanley, PhD Children’s National Hospital, USA | Sandeep Soni, MD CRISPR Therapeutics, USA |
2. Emerging trends in COVID-19 treatment: learning from inflammatory conditions associated with cellular therapies
Chaired By:
Ivan Martin, PhD |
3. International Society for Extracellular Vesicles and International Society for Cell and Gene Therapy statement on extracellular vesicles from mesenchymal stromal cells and other cells: considerations for potential therapeutic agents to suppress coronavirus disease-19
4. Cell-based therapies for coronavirus disease 2019: proper clinical investigations are essential
Co-Chaired By:
| Dominic Clarke, PhD HemaCare, NC, United States | John Fink, MBA Pall Corporation, MA, United States |
5. Transitioning from development to commercial: risk-based guidance for critical materials management in cell therapies
The ISCT scientific community operates on a global scale, providing expert review and support in the interest of proven and safe research practices.
Through liaison work with standards organizations, a broader scientific community, and with investigatory stakeholders, ISCT conducts thought leadership in the field of cell and gene therapy on a global scale. Below is a snapshot of some actions taken by ISCT committees over the past year:
International Council for Commonality in Blood Banking Automation
1. Joint publication with the MSC Committee
International Standards Organization*
2. Standards for hBM-MSC Banking
3. Standards for hUC-MSC Banking
*ISCT has provided ongoing liaison work for two biobanking standards documents, providing consultation around nomenclature for the international standards organization
International Society for Extracellular Vesicles
4. Consultation from the ISCT Presidential Task Force on Unproven/Unethical Cell & Gene Therapies
World Health Organization (WHO)
5. ISCT and the International Nonproprietary Names (INN) Programme have been collaborating on cell and gene therapy standardization efforts of the WHO
Consultations with International Organizations
Since 2004, ISCT has been the host coordinating over 15 stakeholder organizations in the annual FDA Cell Therapy Liaison Meeting (CTLM). These closed meetings enable the cell and gene therapy community to inform the FDA of specific concerns, challenges and recent developments to advance the regulatory field.
Each year, the CTLM report provides valuable coverage of key topics for regulatory consideration, summarizing the presentations that took place at the meeting, and providing key takeaways that are particularly useful to sector stakeholders.
ISCT offers regulatory review and consultation from cell and gene therapy experts for regulatory authorities worldwide.
Review and recommendations are made by our global task force, as well as by regional Legal and Regulatory Affairs Committees in our North America, Europe, and Australia and New Zealand regions. Each consultation consists of significant comments, suggested revisions, and edits for regulatory documentation. Below are the major guidance reviews in 2020:
Australian Parliament
3. Comments on the TGA review of TGO88
4. Response to Parliamentary Inquiry on Approval Processes
Regulatory Consultations and Supports
The International Society for Cell and Gene Therapy (ISCT) and AABB collaborated to form the ISCT-AABB Joint Working Group (WG) following an in-person introduction at the International Cord Blood Symposium in San Francisco in June 2014. The group meets monthly to collaborate on projects that will benefit the membership of both organizations. The WG is comprised of four representatives from each organization, including one Board of Directors’ member, two at-large members and one ex officio staff member in a non-voting role.
The Working Group is charged with the development of projects of common interest with the committee chairs of AABB’s Cellular Therapy Section Coordinating Committee (CTSCC) and ISCT’s standing committees
For 2020, the Joint Working Group has two primary project teams:
- Cellular Therapy Product Stability Program
Expected Completion – January 2021
Led by Karl Stasko and Joan Woeltz, this project aims to educate the community regarding consideration for cellular therapy product stability programs. The project team aims to publish reference documents and/or other communication materials to help with the design of a stability program for HPC products specifically.- A stability survey was conducted with ISCT and AABB membership input
- The report will be published in the ISCT Telegraft and AABB CellSource newsletters
- GTP Interpretative Tool
Expected Completion – September 2021- Led by Wade Atkins, this project aims to establish a GTP interpretative tool that can assist with clarification of HCT/P regulations under 21 CFR 1271 and Section 361 of the PHS Act and/or the FD&C Act

Joseph Schwartz, MD, MPH
Co-Chair, ISCT Lab Practices Committee
USA
“As cellular therapy processing expands, the LPC will provide resources for laboratories to help them reach the highest standards. We aim to make these resources as accessible as possible, including webinars, news briefs and educational sessions incorporated in the annual meeting program.”
The ISCT Lab Practices Committee (LPC) is one of the longest standing committees that has been at the core of ISCT membership since its earliest days. The LPC creates key resources and publications to provide ISCT members with the tools they need to build knowledge in their own laboratories and teams. LPC resources provide practical and up-to-date information for member use, and are created by members, for members.
In 2020, the LPC focused on practical resources for the development and maintenance of cell therapy production at standards of excellence. From practical guidelines on operations and staffing, to specific steps on the commissioning of clean rooms and conducting process validation, the LPC provided focused and relevant content to address emerging needs for cellular therapy laboratories in particular.
In 2020, we welcome Dr. Joseph Schwartz, a medical specialist and leader in transfusion and cellular therapy, as co-chair of the LPC committee.

Anthony Ting, PhD
Chief Commercialization Officer, ISCT
USA
Bringing together a diverse membership to advance CGT
“To continue to be a leader, ISCT needs to identify the critical needs of our industry and use our unique membership of academia, industry and regulatory bodies to provide solutions”
“As the decade continues, we will see improvements in technologies (e.g. gene modification) and expansion of cell and gene therapy to multiple cell types and indications that have not previously been considered,” he says. “During my tenure as Chief Commercialization Officer, I hope to increase the exposure of the early-stage professionals (ESPs) to industry leaders, to expand our membership to include more members of large pharma, to drive technologies that will further enable cell and gene therapy, and most importantly, to consider the patient experience.”
The Chief Commercialization Officer (CCO) is a role within ISCT that provides strategic oversight on the positioning of the Society, as an effective and critical voice in the cell and gene therapy industry. The CCO ensures the Society addresses the needs of Industry members.
In a year in which cell and gene therapies have experienced record public interest and increased funding at a global level, we welcome Dr. Anthony Ting, an established translational leader with successful experience overseeing global product development, as CCO.
“There has been an expansion of other societies and the entrance of large pharma in the cell and gene space,” he observes. “To continue to be a leader, ISCT needs to identify the critical needs of our industry and use our unique membership of academia, industry and regulatory bodies to provide solutions.”
The acceleration of cell and gene therapies means that consensus on the critical issues facing stakeholders with a breadth of interests becomes increasingly important. There is an increased need for dialogue between the diverse member groups within the society, and the interests they represent to enable therapies to reach the market.
ISCT is poised to conduct this kind of dialogue, and Dr. Ting sees a proven model that can be expanded. “In a relaxed environment with ISCT industry members, we are allowed ample time and access to key players in the space and one can truly see how the camaraderie of ISCT enables the growth of our industry,” remarks Dr. Ting. “Some of my fondest memories of ISCT are the Industry Networking receptions that took place at the British Ambassador’s residence in France, and at the House of Parliament in London. I can speak personally to the value of this kind of global industry networking in the Society, and I can see it extending beyond, engaging all of our member groups.”
Each year, the ISCT Commercialization Committee brings together key opinion leaders in the cell and gene therapy industry for its Commercialization Signature Series, to identify key issues, and strategies to address them, for the field to successfully advance. With expert perspectives and timely, practical examples, these meetings produce sector-wide insights that are of benefit across the translational chain.
In 2020, the topic of the Signature Series was “Clinical Breakthroughs: Transforming the Standard of Care”, with a focus towards navigating the development of upcoming cell and gene therapies.

- Best practices for successful phase III clinical trials
- Making the right choices for Trial Patients, CRO, and Site
- Going global with partners in CGT trials
- Tracking patient outcomes: lifelong safety and efficacy of treatments
- Upcoming advancements in CGT research topics and methods
- Identifying the right milestones for successful commercialization
- Addressing the “Innovation Gap”
 | Spearheaded by the ISCT Business Models & Investment Subcommittee, the ISCT Investigators to Investors (I to I) program was created in 2018 with a goal to bridge the knowledge gap between investigators and investors within the cell and gene therapy (CGT) sector. The program strives to educate investigators on how to successfully attract investor attention and capital, as well as support investors in conducting diligence and understanding opportunities within the CGT industry. Ultimately, the I to I program engages these two stakeholder groups in critical conversations that advance the development and delivery of cell and gene therapies. In 2020, the I to I program published a key paper following up on the results of an investment stakeholder survey in the cell and gene therapy industry, conducted in partnership with Bloomberg. This paper, published on an open access basis, provides significant insights into the challenges facing companies in the sector seeking to engage investors, while forecasting trends for capital investment in CGT on a comparative basis to biopharmaceutical investments. The I to I program has also been the driving force behind the ongoing Partnering in Japan series of webinars, developing and sponsoring a webinar in December 2020, focusing on the key lessons learned by leaders in Cell and Gene Therapy in the wake of the Japanese PMDA Conditional Approvals Act. |

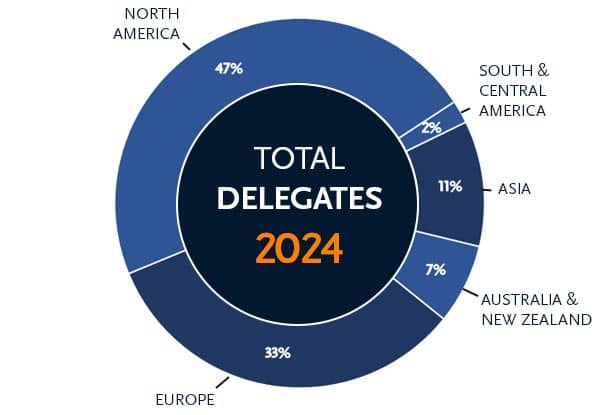
Membership: 50% outside of the United States from 60+ countries
Co-Founded Organizations


Strategic Liaisons





















Webinar Sponsors








Joint Session Partners









Co-Founded Organizations


Strategic Liaisons




















Webinar Sponsors








Joint Session Partners









Connecting to a Virtual World
Since 1992, ISCT has connected more than 19,000 delegates through our Annual Meetings and communicated with more than 35,000 cell and gene therapy professionals at cutting-edge meetings, events, webinars and seminars to translate the advancement of research into clinical adoption and standard of care over the past 26 years.
This year, ISCT was faced with the unprecedented challenge of delivering a Virtual Annual Meeting. Under the leadership of the meeting co-chairs and the organizing committee, ISCT was able to leverage a cutting-edge virtual platform and an exciting scientific program together to bring more than 2000 delegates together at ISCT 2020 Paris Virtual.
ISCT 2020 Paris Virtual
Chaired By:

Ivan Martin, PhD
University Hospital Basel, CHE

Rachele Ciccocioppo, MD
University of Verona, ITA

Christian Chabannon, MD, PhD
Institut Paoli-Calmettes, FRA
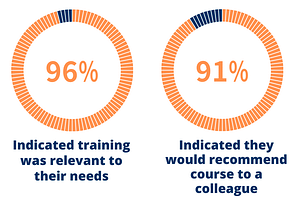
ISCT ESP Mentoring Program 2020 – 2021
A Record-Breaking Kick-Off
The Early Stage Professionals (ESPs) Mentoring Working Group launched the 2020-2021 Edition of the ISCT Mentoring Program with two kick-off calls on November 19 and 20 for Asia, Australia New Zealand and North, South & Central America and Europe. These interactive kick-off calls featured WebEx breakout rooms with over 80 attendees, the largest single meeting on this platform within ISCT. Mentors and mentees were able to introduce themselves and begin the networking process.
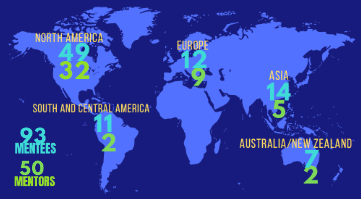
The third official round of the program (4th edition including our 2016 pilot), themed “Expose + Expand” is our biggest program to date with 38 mentors and 75 mentees, representing 26 countries across all 5 ISCT regions. Expertise-driven matching was achieved within all five expertise areas, including Research, Clinical, Industry and Commercialization, Regulatory, and Laboratory & Operations.

Diana Cirstea, MD
Harvard Medical School
USA
I would like to express my gratitude to your program and to my mentor Dr. Sandeep Soni. If I were to describe in one word my early experience with Dr. Soni, this word would be EMPOWERING. It’s exactly what I was hoping for.
Aisha Khan Albert Ribickas Anand Srinivasan Andrés Caicedo Anthony Ting Antonio Carlos Campos de Carvalho Bruce Levine Bryan Choi Cheryl Cox Cornelia Kasper | Daniel J. Weiss Debora Barton Dominic Wall Anna Krasnodembskaya Edwin Wagena Erin Rasch Fermin Sanchez-Guijo Isaac Godfroy J. Wade Atkins Jacques Galipeau | Janet Macpherson Jennifer Joe Joseph Schwartz Karen English LaTonya Hickson Lindsay Davies Lisa Kretzschmar Manal Morsy Melinda Caltabiano
| Xiaokui Zhang Yufang Shi Yvette Tanhehco Mitchell S. Cairo Nadim Mahmud Sandeep Soni Sandhya R. Panch Sowmya Viswanathan
|
Aisha Khan Albert Ribickas Anand Srinivasan Andrés Caicedo Anthony Ting Antonio Carlos Campos de Carvalho Bruce Levine Bryan Choi Cheryl Cox Cornelia Kasper Daniel J. Weiss Debora Barton Dominic Wall Anna Krasnodembskaya Edwin Wagena Erin Rasch Fermin Sanchez-Guijo Isaac Godfroy J. Wade Atkins Jacques Galipeau | Janet Macpherson Jennifer Joe Joseph Schwartz Karen English LaTonya Hickson Lindsay Davies Lisa Kretzschmar Manal Morsy Melinda Caltabiano Xiaokui Zhang Yufang Shi Yvette Tanhehco Mitchell S. Cairo Nadim Mahmud Sandeep Soni Sandhya R. Panch Sowmya Viswanathan |


The Inaugural ISCT Master Programme Scholarships
Made possible by Miltenyi Biotec, and in partnership with the Andalusian Government and University of Granada, the ISCT European Regional Executive Committee launched the inaugural round of the ISCT Master Programme Scholarships.
Three scholarships were made available for ISCT members in Europe with professional interests in Good Manufacturing Practices (GMP) to gain proficiency with GMP guidelines, laboratory practices, and intensive theoretical training focused on applying GMP to advanced therapy medicinal products (ATMPs). Each scholarship provides funding to undergo a Master’s Degree in Manufacturing of ATMPs, specializing as a Qualified Person, Manufacturing Manager, or Quality Control Manager
2020 Scholarship Awardees

Alejandro Barquero, MPharm, MSc
Advent Bioservices Ltd
United Kingdom
Master Degree in Manufacturing of Advanced Therapy Medicinal Products, specialization as Qualified Person
Alejandro Barquero holds a Master of Pharmacy and an MSc in Advanced Therapies & Biotechnological Innovation. In 2019, Alejandro joined Advent Bioservices Ltd, biopharmaceutical CDMO specialized in ATMPs, where he has been working as part of the Quality Assurance team ever since.
At the time of completing this Master Degree programme with a specialization as Qualified Person, Alejandro looks forward to being in the position to meet the statutory requirements to assume a QP role within his organization and help in the development and commercialization of ATMPs.

Miriam López-Parra, MD
University Hospital of Salamanca
Salamanca, Spain
Master Degree in Manufacturing of Advanced Therapy Medicinal Products, specialization as Qualified Person
Miriam López Parra is a Hematologist and Qualified Person of the GMP Facility of the University Hospital of Salamanca, and researcher at the Institute of Biomedical Research of Salamanca.
Miriam plans to apply this Master’s degree to tackling one of the most important challenges she will have in the coming years, which is the development of a new Cellular Production Unit in her Hospital.

Katja Sirviö, MSc (Pharm)
Kuopio Center for Gene and Cell Therapy
Finland
Master Degree in Manufacturing of Advanced Therapy Medicinal Products, specialization as Manufacturing Manager
Katja Sirviö has 20 years experience working in academic and ATMP industry. She has experience on stem cell research in neurodegenerative diseases at the University of Eastern Finland as well as developing novel cell therapies for solid cancers in Finvector and Kuopio Center for Gene and Cell Therapy.
The Master in Manufacturing of Advanced Therapy Medicinal Products will provide the essential knowledge for Katja to participate in bringing future therapies to the patients.
ISCT in the Public Eye
ISCT is a powerful and visible voice for the Cell and Gene therapy sector, represented in the media through press releases and key coverage. In the public space, ISCT leaders speak to well-supported and ethical positions across the translational spectrum from early-stage clinical research to the authorization and marketing of viable products, while providing a clear vision on the ongoing developments in the field.
Press Releases
September 14, 2020
ISCT issues key findings and recommendations following 2020 Annual Meeting for cell and gene therapy
May 11, 2020
ISCT demands proper clinical investigation for COVID-19 therapies
March 25, 2020
ISCT takes a stand against organizations marketing unproven COVID-19 cell and gene therapies
October 21, 2019
ISCT forms cell and gene therapy sector-wide coalition combating rise of unproven commercial cell banking services
November 18, 2018
ISCT publishes annual report on cell and gene therapy market authorizations
Media Features
ISCT Webinars in 2020
February 6
Total registered: 226

Organized by the ISCT Immuno & Gene Therapy Committee
July 8
July 30
August 26
Total registered: 131

Organized by the ISCT Asia Regional Executive Committee
October 6
Total registered: 141

Organized by the
ISCT Business Models & Investment Subcommittee
October 21
Total registered: 321

Organized by the ISCT Process and Product Development Subcommittee
November 18
December 2
Total registered: 72

Organized by the
ISCT Business Models & Investment Subcommittee
To address patient uncertainty around cell and gene therapy treatments throughout the explosive growth of the field, ISCT’s Presidential Task Force (PTF) on the Use of Unproven and/or Unethical Cell and Gene Therapies works to provide reliable and verified information to a global public.
Since its inception, the PTF has consolidated several up-to-date resources for the public regarding the field of cell and gene therapy.
Throughout this past year, ISCT and the PTF have taken a stand against the marketing of unproven COVID-19 cell and gene therapies, and demanded that proper standards be upheld in clinical investigation for COVID-19 therapies.
The PTF has acted as a visible and consistent conduit between the cell and gene therapy sector and the public, speaking out to emerging issues in unproven cell therapies, and highlighting best practices for patients to ensure that stem cell therapies they are offered are safe, proven, and ethical. The PTF is composed of representatives from academia, regulatory, and industry, and aims to build consensus on issues that span across the field of cell and gene therapies.
CGT Market Authorization – Updated for 2020
The ISCT CGT Market Authorization Report is a regularly updated document that will be republished yearly and available online.
This report is the result of an increase in the number of CGT market authorizations, as well as an increase in unproven approaches where cells are delivered as treatments without rigorous scientific and regulatory assessment, and authorization. This resource aims to help practitioners and patients to better understand what is on the market for cell and gene therapies. This report ultimately allows practitioners and patients to better make informed decisions, to access proven, ethical, and licensed cell and gene interventions approved by a regulatory or medical agency.
ISCT is a member of the National Academies of Sciences Engineering Medicine’s Forum on Regenerative Medicine. The forum brings together academia, industry, government, patient and provider organizations, regulatory bodies, foundations, societies, associations, and other groups, to discuss the challenges and opportunities of regenerative medicine, potentially improving the health of millions of people worldwide through the development of effective new therapies.
Representing us on the forum are: Dr. Daniel Weiss, Karen Nichols, Dr. Catherine Bollard, Dr. Bruce Levine, and Dr. Patrick Hanley.
2020 ISCT Career Achievement Award Winner
Past President, ISCT
Fortitude and Flexibility
Dominating the Medicine Maker “Top 20 for 2020” List for Advanced Medicines
Celebrating 9 ISCT Leaders moving the field forward
ISCT Awards 2020
Recognizing the Achievements and Contributions of Our Membership
The 2020 Dennis Confer Innovate Award
Honoring a Visionary in the Field
A Journey of Fortitude and Flexibility

“Seeing the difference that mentors made to my career and that of my wife taught and inspired me throughout my own efforts to mentor newcomers to the field.”
Dr. Brenner is a premier clinical scientist whose career has been marked by a passion for excellence in research which was displayed during his leadership in some of the most advanced institutions globally in cell and gene therapy. Parallel to his scientific ambitions, Dr. Brenner also has a distinguished history of mentorship, most recently recognized through an award from the American Society of Hematology, and which can be attested to through an ever-growing list of mentees leading advancements in cell and gene therapy. Dr. Brenner’s notable contributions also include effective leadership in his roles as former president of both ISCT and the American Society for Gene & Cell Therapy (ASGCT).
The road to this point hasn’t always been smooth for Dr. Brenner – he has overcome some major obstacles along the way.
A globally leading publication on drug development, The Medicine Maker, distinguished several notable ISCT members on its 2020 Power List.
This year, the Power List focused on three specific sectors: Small Molecules, Advanced Medicine, and Biopharmaceuticals.
Many ISCT Leaders were featured in the Advanced Medicines segment of this list, showcasing their contributions to driving this industry forward and making life-saving developments on a global scale.
Bruce Levine, PhD
President (2020-2022)
International Society for Cell and Gene Therapy
Carl June, MD
Richard W. Vague Professor in Immunotherapy
University of Pennsylvania
Claudia Zylberberg, PhD
Co-Founder, Chief Executive Officer and President
Akron Biotechnology
Kurt Gunter, MD, FASCP
Past President (2016 – 2018)
International Society for Cell and Gene Therapy
Miguel Forte, PhD
Past Chief Commercialization Officer (2018 – 2020)
International Society for Cell and Gene Therapy
Massimo Dominici, MD
Past President (2014-2016), and President’s Task Force Chair
International Society for Cell and Gene Therapy
Qasim Rafiq, PhD
Associate Professor
University College London
Queenie Jang, MBA
Chief Executive Officer
International Society for Cell and Gene Therapy
Usman “Oz” Azam, MD
President and Chief Executive Officer
Tmunity Therapeutics
A globally leading publication on drug development, The Medicine Maker, distinguished several notable ISCT members on its 2020 Power List.
This year, the Power List focused on three specific sectors: Small Molecules, Advanced Medicine, and Biopharmaceuticals.
Many ISCT Leaders were featured in the Advanced Medicines segment of this list, showcasing their contributions to driving this industry forward and making life-saving developments on a global scale.
Bruce Levine, PhD
President (2020-2022)
International Society for Cell and Gene Therapy
Carl June, MD
Richard W. Vague Professor in Immunotherapy
University of Pennsylvania
Claudia Zylberberg, PhD
Co-Founder, Chief Executive Officer and President
Akron Biotechnology
Kurt Gunter, MD, FASCP
Past President (2016 – 2018)
International Society for Cell and Gene Therapy
Miguel Forte, PhD
Past Chief Commercialization Officer (2018 – 2020)
International Society for Cell and Gene Therapy
Massimo Dominici, MD
Past President (2014-2016), and President’s Task Force Chair
International Society for Cell and Gene Therapy
Qasim Rafiq, PhD
Associate Professor
University College London
Queenie Jang, MBA
Chief Executive Officer
International Society for Cell and Gene Therapy
Usman “Oz” Azam, MD
President and Chief Executive Officer
Tmunity Therapeutics
Award Showcase
Click on any trophy to see full details on the award and recipient

Top Scoring Abstract Award
Ryang Lee, PhD
Texas A&M College of Medicine; CellCue LLC
United States
TWIST1 and TSG6 as Potency Biomarkers of Human MSCs in Pre-clinical Disease Models
Early Stage Professional Award
Hannah Song, PhD
National Institutes of Health
United States
CAR-T cell expansion platforms yield distinct phenotypic and transcriptional profiles
Award Sponsored by ISCT
Early Stage Professional Award
Claudia Manriquez Roman, MSC
Mayo Clinic
United States
TNFR2 as a target to improve CD19-directed CART cell fitness and antitumor activity in large B cell lymphoma
Award Sponsored by ISCT
Early Stage Professional Award
Shruthi Pandi Chelvam, BE
Singapore MIT Alliance for Research and Technology
Singapore
Anomaly Detection for Microbial Contamination in Mesenchymal Stromal Cell Culture
Award Sponsored by ISCT
ESP Rookie of the Year
Hugo Sugier, MSc
Aenitis Technologies; INSERM UMR-MD 1197
France
Characterization of the Acoustic Contrast Factor: a New Approach for a Label-Free Separation of the Stromal Vascular Fraction
Award Sponsored by Cytotherapy
Top Scoring Canadian Abstract Award
JonDavid De Jong, PhD
Virica Biotech Inc.
Canada
Overcoming Barriers in Viral Vector Manufacturing: Small Molecule Targeting of Antiviral Defences
Award Sponsored by Stem Cell Network Canada
Top Scoring Emerging Economies Abstract Award
Pawan Gupta, MBBS, MD, DNB, PhD
FOSCAL
Colombia
Security and Efficacy of Intradermal Injection of Mesenchymal Stem Cells Derivatives on Chronic Diabetic Foot Ulcers: A Randomized Controlled Clinical Trial
Award Sponsored by Cytotherapy
Best Poster Award
Kajal Chaudry, PhD
Children’s National Medical Center
United States
B7H3-CAR NK cells and DNR co-transduced NK shows maintain their potency against TGF-B mediated immune suppression
Award Sponsored by BuioCanRx
Elevator Pitch Abstract Award
Andrea Vervoort, BASc
Virica Biotech Inc.
Canada
Bioprocess Modelling of Viral Sensitizer(TM) Mediated Yield Enhancement on Upstream Viral Vector Production
Award Sponsored by Cytotherapy
Technologist Award
Anastasia Papadopoulou, PhD
George Papanikolaou Hospital
Greece
Safety and Efficacy of SARS-CoV-2-Specific T Cells as Adoptive Immunotherapy for High-Risk COVID-19 Patients: a Phase I/II, Randomized Clinical Trial
Award Sponsored by ISCT
Technologist Award
Mercy Gohil, B.Sc
University of Pennsylvania Perelman School of Medicine
Unied States
Large-scale manufacture of CAR T cells engineered with augmented proliferative capacity and function via 3 day process
Award Sponsored by ISCT
Technologist Award
Christian Tebid Tebid, PhD(c)
Centre de recherche l’Hôpital Maisonneuve-Rosemont (CR-HMR), Université de Montréal
Canada
Mesenchymal Stromal Cells Secretome-Induced Trabecular Meshwork Regeneration for Glaucoma Therapy
Award Sponsored by ISCT
Orthopedics & Musculoskeletal Therapies Abstract Award
Daniela Bueno,Daniela Bueno, DDS, PhD
RCRIO/ANADEM/HOSPITAL MUNICIPAL INFANTIL MENINO JESUS; Hospital Sírio-Libanês
Brazil
Bone Tissue Engineering for Cleft Lip and Palate Patients: A Multicenter Clinical Trial
Award Sponsored by ISCT South and Central America Regional Executive Committee
Orthopedics & Musculoskeletal Therapies Abstract Award
Paulo Martins, PhD
QIMR Berghofer Medical Research Institute
Australia
Ephrin receptor A3–targeted CAR T cell immunotherapy for glioblastoma
Award Sponsored by ISCT Australia and New Zealand Regional Executive
2021 Career Achievement Award in Cell & Gene Therapy

2021 Darwin J. Prockop Mentoring Award

ISCT Top Scoring Stem Cell Engineering Abstract Award
Li Xu, MSc
Duke University
United States
Human Umbilical Cord Blood Derived Cell Therapy Product, DUOC-01, Promotes Remyelination by Driving the Differentiation of OPC
Sponsored by ISCT Stem Cell Engineering Committee
ISCT President Dr. Bruce Levine received this year’s Dennis Confer Innovate Award, presented annually by Be The Match to an individual who has explored new ways to improve transplant outcomes or experiences for patients, donors and providers.
This prestigious award was presented to Dr. Levine in recognition of his pivotal role in the development of the first successful CAR T Cell-based therapy, and the outstanding impact that this development has had upon the lives of patients and their families.
See the video from Be The Match featuring perspectives from Dr. Levine, colleagues who have worked with him, and several patients whose lives have been saved by the contributions of Dr. Levine and his teammates.
ISCT acknowledges the service of its members, volunteering expertise, time, and energy to the vast task of advancing the field of cell and gene therapies.
From mentoring the next generation to publishing key standards and fostering relationships across the sector, ISCT members set the gold standard.
Thank you for being part of the ISCT community
We acknowledge these Society leaders who have completed terms of service in a formal position in 2020.
Your hard work and dedication have continually pushed the society to new heights, and we cannot wait to re-connect soon:
Board of Directors

John Rasko, AO, MBBS, PhD, FRCPA, FRACP, FAHMS
President
June 2018 – June 2020
Royal Prince Alfred Hospital
Sydney, Australia

Catherine Bollard, MBChB, MD
Chair, Strategic Advisory Council (Immediate Past President)
June 2018 – June 2020
Children’s National Hospital
Washington, DC, United States

Ngaire Elwood, PhD
Australia & New Zealand, Regional Vice-President
June 2018 – June 2020
Murdoch Children’s Research Institute
Melbourne, Australia

Lynn O’Donnell, PhD
North America, Regional Vice-President
June 2018 – June 2020
Ohio State University, James Cancer Hospital
Columbus, OH, United States

Miguel Forte, MD, PhD
Chief Commercialization Officer
June 2016 – June 2020
Bone Therapeutics
Brussels, Belgium

Nadim Mahmud, MBBS, PhD
Elected Member PhD
June 2018 – June 2020
University of Illinois College of Medicine
Chicago, IL, United States

Jeannette Bloom, MBA, MT(ASCP)SBB
Elected Member Technologist
June 2018 – June 2020
Baylor College of Medicine
Houston, TX, United States
Committee Chairs

Rosemarie Bell, B.App.Sc Micro/Biochem MASM
Co-Chair, LPC Committee
June 2018 – June 2020
QGen Cell Therapeutics
QIMR Berghofer Medical Research Institute
Queensland, Australia

Nancy Collins, PhD
Senior Editor, Telegraft
June 2018 – June 2020
University of Toledo
Toledo, OH, United States

Bill Milligan
Co-Chair, BM&I Subcommittee
June 2013 – Nov 2020
Stemiment Biotherapeutics
Vancouver, Canada

Kenneth Micklethwaite, MBBS, PhD, FRACP, FRCPA
Co-Chair, Immuno & Gene Therapy Committee
Jan 2018 – Jan 2020
Westmeade Institute for Medical Research
New South Wales, Australia

Jacques Galipeau, MD
Chair, MSC Committee
June 2017 – June 2020
University of Wisconsin-Madison
Madison, WI, United States
Regional Officers

Pawan Kumar Gupta, MBBS, MD, DNB, PhD
Secretary, Asia Regional Executive Committee
June 2017 – June 2020
Stempeutics
Bangalore, India

Melissa Lin, PhD
Treasurer, Asia Regional Executive Committee
June 2017 – June 2020
National Research Foundation Singapore
Singapore

Mohamed Abou-El Enein, MD, PhD
Secretary, Europe Regional Executive Committee
June 2017 – June 2020
Charité Medical University in Berlin
Berlin, Germany

Britta Eiz-Vesper, PhD
Treasurer, Europe Regional Executive Committee
June 2017 – June 2020
Hannover Medical School
Hannover, Germany

Patrick Hanley, PhD
Treasurer, North America Regional Executive Committee
June 2017 – June 2020
Children’s National Hospital
Washington, DC, USA

Jhann Arturo, MD, PhD, MSc
Secretary, South & Central America Regional Executive Committee
June 2017 – June 2020
Immugen Corporation
Colombia

Vicki Antonenas, BSc, MSc
Secretary, Australia and New Zealand Regional Executive Committee
June 2017 – June 2020
Westmead Hospital
Sydney, Australia
ISCT acknowledges the service of its members, volunteering expertise, time, and energy to the vast task of advancing the field of cell and gene therapies.
From mentoring the next generation to publishing key standards and fostering relationships across the sector, ISCT members set the gold standard.
Thank you for being part of the ISCT community
We acknowledge these Society leaders who have completed terms of service in a formal position in 2020.
Your hard work and dedication have continually pushed the society to new heights, and we cannot wait to re-connect soon:
Board of Directors

John Rasko, AO, MBBS, PhD, FRCPA, FRACP, FAHMS
President
June 2018 – June 2020
Royal Prince Alfred Hospital
Sydney, Australia

Catherine Bollard, MBChB, MD
Chair, Strategic Advisory Council (Immediate Past President)
June 2018 – June 2020
Children’s National Hospital
Washington, DC, United States

Ngaire Elwood, PhD
Australia & New Zealand, Regional Vice-President
June 2018 – June 2020
Murdoch Children’s Research Institute
Melbourne, Australia

Lynn O’Donnell, PhD
North America, Regional Vice-President
June 2018 – June 2020
Ohio State University, James Cancer Hospital
Columbus, OH, United States

Miguel Forte, MD, PhD
Chief Commercialization Officer
June 2016 – June 2020
Bone Therapeutics
Brussels, Belgium

Nadim Mahmud, MBBS, PhD
Elected Member PhD
June 2018 – June 2020
University of Illinois College of Medicine
Chicago, IL, United States

Jeannette Bloom, MBA, MT(ASCP)SBB
Elected Member Technologist
June 2018 – June 2020
Baylor College of Medicine
Houston, TX, United States
Committee Chairs

Rosemarie Bell, B.App.Sc Micro/Biochem MASM
Co-Chair, LPC Committee
June 2018 – June 2020
QGen Cell Therapeutics
QIMR Berghofer Medical Research Institute
Queensland, Australia

Nancy Collins, PhD
Senior Editor, Telegraft
June 2018 – June 2020
University of Toledo
Toledo, OH, United States

Bill Milligan
Co-Chair, BM&I Subcommittee
June 2013 – Nov 2020
Stemiment Biotherapeutics
Vancouver, Canada

Kenneth Micklethwaite, MBBS, PhD, FRACP, FRCPA
Co-Chair, Immuno & Gene Therapy Committee
Jan 2018 – Jan 2020
Westmeade Institute for Medical Research
New South Wales, Australia

Jacques Galipeau, MD
Chair, MSC Committee
June 2017 – June 2020
University of Wisconsin-Madison
Madison, WI, United States
Regional Officers

Pawan Kumar Gupta, MBBS, MD, DNB, PhD
Secretary, Asia Regional Executive Committee
June 2017 – June 2020
Stempeutics
Bangalore, India

Melissa Lin, PhD
Treasurer, Asia Regional Executive Committee
June 2017 – June 2020
National Research Foundation Singapore
Singapore

Mohamed Abou-El Enein, MD, PhD
Secretary, Europe Regional Executive Committee
June 2017 – June 2020
Charité Medical University in Berlin
Berlin, Germany

Britta Eiz-Vesper, PhD
Treasurer, Europe Regional Executive Committee
June 2017 – June 2020
Hannover Medical School
Hannover, Germany

Patrick Hanley, PhD
Treasurer, North America Regional Executive Committee
June 2017 – June 2020
Children’s National Hospital
Washington, DC, USA

Jhann Arturo, MD, PhD, MSc
Secretary, South & Central America Regional Executive Committee
June 2017 – June 2020
Immugen Corporation
Colombia

Vicki Antonenas, BSc, MSc
Secretary, Australia and New Zealand Regional Executive Committee
June 2017 – June 2020
Westmead Hospital
Sydney, Australia
Cytotherapy®, the official journal for the International Society for Cell and Gene Therapy (ISCT), publishes novel and innovative results from high quality scientific and clinical studies in the fields of cell and gene therapy.
Across 2020, the journal continued the re-branding efforts from 2019, expanding its team with the addition of Dr. Patrick Hanley as Commissioning Editor, and introducing a revised scope and focus. Cytotherapy has maintained a strong position to advance and expand the field of cell and gene therapy for clinical researchers, oncologists, hematologists, physicians, and regulatory experts alike. The Journal had a 4.218 impact factor for 2019, and is poised to continue to grow.
Take a look at our Talking with Giants series, launched in 2019, which showcases leaders in the field of cell and gene therapy through informal question and answer articles. Talking with Giants is featured at the start of each issue of Cytotherapy.
You can read issues of Cytotherapy here.
89 Published Papers – 468 Conference Abstracts


Janet Macpherson, PhD
Cytiva
Australia

C. Russell Y. Cruz, MD, PhD
Children’s National Medical Center
USA
Available to ISCT members and the cell and gene therapy community around the world, Telegraft is the Society’s bi-monthly e-newsletter and your go-to resource to keep up to date with the latest Society activities and important developments in cell and gene therapy. The Telegraft Editorial Board compiles robust issues aiming to enhance communication and understanding between all Society constituencies: academic, clinical, basic and translational science, regulatory, technical, and commercial.
In 2020, leadership of the Telegraft was passed to Dr. Janet Macpherson and Dr. Russell Cruz. As co-editors of the Newsletter, Drs. Macpherson and Cruz aim to further align the content to the needs of the growing ISCT membership, and to provide an accessible and effective window into the breadth and depth of ISCT.
Thank you, Dr. Nancy Collins, for your leadership
Telegraft developed the Telegraft Hub, an ISCT community page, and introduced several new article lines focused on the scientific arm of the Society. Readership for the newsletter has grown consistently across volume 27 (2020) and is forecasted to continue on this trajectory.
Telegraft Hub
Membership to Meet Your Needs
ISCT members are the core of our Society. With member representation at every step of the translational pathway, ISCT serves to support your unique needs as we advance the CGT field together. ISCT offers tailored membership categories for Individuals, Labs, and Industry Companies – Find the membership best suited for you.
- Reduced rates at ISCT-sponsored events, including webinars, technical workshops, research and clinical symposia, and the ISCT meetings.
- Access to members-only web resources, including the ISCT Member Networking Database and online discussion boards.
Meetings – Save The Date
“Orchestrating Global CGT Translation – Building Consensus for the Path Forward”
The Newly Updated ISCT Career Center
Recruiting, or looking to be recruited?
Visit the new ISCT Career Center. As part of our 2020 vision, we have continued to ensure that our job postings engage and help laboratories worldwide to expand as they seek qualified technicians, scientists, and business professionals. If you manage or are part of a lab, please inquire about our efforts to support your growth through catered job postings and outreach.
Our Volunteer Center
The ISCT Volunteer Center is the new hub designed to facilitate members seeking to join one of our 43 standing committees, or to contribute to our bimonthly e-newsletter, Telegraft.
If you have a passion for the advancement of the field of cell and gene therapy, and you wish to contribute your voice towards shaping the field by sharing your expertise and experience, please check in regularly to the volunteer center, as opportunities are expected to expand.
Our Committees
ISCT has 43 stakeholder committees, working in a number of scientific, commercial and regulatory disciplines within cell and gene therapy. Joining an ISCT committee is an invaluable networking opportunity, allowing you to connect with cell and gene therapy professionals from all over the world. If you would like to bolster your career by joining an ISCT committee, visit our Committees page to see how.
Showcase Your Research:
 Cytotherapy
Cytotherapy
Cytotherapy, official journal of the International Society for Cell & Gene Therapy (ISCT®), publishes novel and innovative results from high quality basic, translational and clinical studies in the fields of cell and gene therapy.
Aims and Scope:
Studies evaluating the potency of experimental cell and gene therapies in clinically relevant animal models of disease and describing important advances in cell/gene-based product manufacturing and validation are welcomed. Results of clinical studies evaluating the safety and efficacy of cell and gene therapies in early and late phase trials are also of interest. In addition to Short Reports and Full-Length Articles, the journal also accepts Editorials addressing emerging trends and potential controversies in the field, and Review articles summarizing bodies of work that have made lasting impacts in the field.
Interested in showcasing your research with Cytotherapy?
Please get in touch with Dr. Patrick Hanley, Commissioning Editor
Telegraft:
Available to ISCT members around the world, Telegraft is the Society’s leading global cell therapy newsletter which updates readers on new cell and gene therapy developments, regulatory updates, and regular columns on related meetings, organizations, and events, summaries of work being done in both academic and industry labs around the world.
If you are interested in contributing an article to Telegraft, or in helping out with our editorial process, please visit here.
Tools for Learning
ISCT has a large number of valuable and comprehensive resources for ISCT members and cell and gene therapy professionals. This toolbox of resources includes a useful glossary of cell therapy terms, the ISCT Cell Therapy Bioprocessing Tools and Reagents Database, a centralized catalogue of available products for use in cell therapy processing, manufacturing, and research, as well as valuable patient resources from the innovative ISCT Presidential Task Force (PTF) on the Use of Unproven and/or Unethical Cell and Gene Therapies website, which was launched in 2018.
Visit the Cell Therapy Community Resources page to learn more.