Kyung-Phil Kim
Abstract: Human Mesenchymal Stromal Cells Administered After Radiotherapy and Surgery in a Soft Tissue Sarcoma Mouse Xenograft Model Do Not Promote Local Recurrence or Metastasis
Abstract: Human Mesenchymal Stromal Cells Administered After Radiotherapy and Surgery in a Soft Tissue Sarcoma Mouse Xenograft Model Do Not Promote Local Recurrence or Metastasis
Abstract: Safety Considerations in the Generation of Clinical Grade Autologous IPS Cell Lines
Abstract: First Trimester Human Umbilical Cord Perivascular Cells Expanded With CGMP Compliant Human Platelet Outperform Conventional MSC Sources As Regenerative Therapy in a Rat Model
ISCT acknowledges the service of its members, volunteering expertise, time, and energy to the vast task of advancing the field of cell and gene therapies.
From mentoring the next generation to publishing key standards and fostering relationships across the sector, ISCT members set the gold standard.
We acknowledge these Society leaders who have completed terms of service in a formal position in 2021.
Your hard work and dedication have continually pushed the society to new heights, and we cannot wait to connect again soon:

John Rasko, AO, MBBS, PhD, FRCPA, FRACP, FAHMS
Chair, Strategic Advisory Council (Immediate Past President)
June 2020 – June 2022
Royal Prince Alfred Hospital
Sydney, Australia

Lizette Caballero, BS, MT(ASCP)
Global Secretary
June 2019 – June 2022
UCSF Blood and Marrow Transplant Lab
San Francisco, CA, United States

Rajiv Khanna, PhD AO
Australia & New Zealand, Regional Vice-President
June 2020 – June 2022
QIMR Berghofer Medical Research Institute
Brisbane, Australia

Mitchell Cairo, MD
North America, Regional Vice-President
June 2020 – June 2022
New York Medical College
New York, NY, United States

Karen Nichols, Esq.
Chief Regulatory Officer
March 2016 – June 2022
Vertex Pharmaceuticals
Cambridge, MA, United States

Vicki Antonenas, BSc, MSc
Elected Member Technologist
June 2020 – June 2022
Sydney Cellular Therapies Laboratory,
Westmead Hospital
Australia

Patrick Hanley, PhD
Co-Chair Immuno-Gene
Therapy Committee
Children’s National Hospital
United States

Giuseppe Orlando, MD, PhD
Co-Chair Gastrointestinal Committee
Wake Forest University
United States

Rachele Ciccocioppo, MD
Co-Chair Gastrointestinal Committee
University of Verona
Italy

George Muschler, MD
Co-Chair Orthopedic & Musculoskeletal Therapies
Cleveland Clinic
United States

Christian Jorgensen, MD, PhD
Co-Chair Orthopedic & Musculoskeletal Therapies
University Hospital Lapeyronie
France

Sarah Chan, Ma PhD
ECGT Committee
University of Edinburgh
United Kingdom

Andrew Hoffman, DVM, DVSc
Exosomes Committee
University of Pennsylvania
United States

John Fink, MBA
Co-Chair Process Development and Manufacturing Committee
PerkinElmer, Inc.
United States

Emily Hopewell, PhD
Indiana University School of Medicine
United States

Aaron Tze Kai Tan
Stanford University
United States

Lizbeth Diaz Polo, MD
University of San Martin de Porres
Spain

Jordan Greenberg, PhD
Shoreline Biosciences
United States

Shabnum Patel, PhD
CARGO Therapeutics
United States

Nancy H. Collins, PhD
University of Toledo
Toledo, OH, United States

Satyam Arora, MD
Super Speciality Paediatric Hospital and Post Graduate Teaching Institute
India

Lab Practices Committee
Vicki Antonenas, BSc, MSc
Westmead Hospital
Australia

Europe Legal & Regulatory Affairs Committee
Paula Salmikangas, PhD
NDA Group
Finland
Building Consensus and Community
Motivated to stay true to the essence of ISCT as a truly global translation-focused community of experts, ISCT made major strategic progress in 2022, with significant investments in community development, workforce development and addressing ethical issues. Working in collaboration with ISCT leadership, the Society enhanced the value of its membership value across all disciplines for leaders and newcomers alike as well as developed key resources and drove consensus to combat the critical issues facing the wider CGT sector. As the ISCT community continues to grow, the Society is perfectly positioned to empower the field to drive the translation of research into safe, effective and accessible treatments with long-lasting impact.

Excited to welcome delegates to the first in-person ISCT meeting post Pandemic, ISCT was determined to make a significant impact with ISCT 2022 San Francisco. Welcoming 1,646 delegates from across the globe, ISCT 2022 was the largest ISCT Annual Meeting to date and was proud to serve as a key event reconnecting the wider CGT community. A standout feature of the meeting was the redesign of the ISCT Scientific program, a strategic initiative inspired to better cater to the interests of all delegates, across the full range of CGT development and deployment.
The Translational Pathway Program, reflects ISCT’s role in driving the translation of scientific research into safe and effective therapies that can be deployed to patients. The new program was designed to mirror the therapeutic development process and integrated perspectives across the CGT sector to address key topics at all stages of translation.
As a counter-part to the Translational Pathway Program, ISCT also launched the Roundtable Program at ISCT 2022. Unique in the cell and gene therapy sector, this program provides a platform for delegates to debate and collaboratively develop consensus and solutions to practical problems and barriers in the field. Recognizing the breadth and depth in expertise of Annual Meeting delegates, ISCT developed this unique platform to connect professionals from across the globe who share the same goals and encounter the same difficulties.
“ISCT broadened its focus, through a whole series of roundtables, to cover wider challenges experienced across the industry, from generating return on investment to managing the supply chain. ISCT will continue to monitor the efficacy of solutions generated at the meeting and continue to work with all stakeholders to ensure an increasing number of patients are able to benefit from cell and gene therapies.”

Bruce Levine, PhD
University Of Pennsylvania
United States
The rapid growth of the sector has introduced new ethical concerns associated with the development, regulatory authorization, and distribution of cell and gene therapies. Recognizing this, ISCT has worked to develop internal infrastructure so that the Society remains a leading voice on critical and topical ethical issues.
Undertaking a broader remit to identify these key ethical issues, ISCT has introduced the Committee on the Ethics of Cell and Gene Therapy (ECGT). Formally the ISCT Presidential Task Force on the Use of Unproven and/or Unethical Cell and Gene Therapies, this new multidisciplinary committee regroups key stakeholder perspectives to promote the ethical development of the sector.
To further refine the Society’s position on ethical issues, ISCT formed the ISCT Expanded Access Working Group. The overarching objective of the Working Group is to further refine its position on ethical issues arising from the use, and potential misuse, of the expanded access pathway, culminating in the development of a code of ethics.
Under the guidance of the new ECGT committee, ISCT is now better positioned to give informed consensus on ethical issues. In 2022, following the 2022 US federal court ruling in favor of California Stem Cell Treatment Center, Inc., and Cell Surgical Network Corporation, ISCT published a press release highlighting the dangers of unproven cell therapies to patient safety.
With Industry members representing a vital part of ISCT DNA, in 2022, ISCT worked to adapt the industry membership program to better meet the needs of our growing community. In collaboration with ISCT Industry leadership, ISCT worked to tailor its Committees using feedback from our members to create a program that supports those who are at the forefront of driving the translation of cell and gene therapies.
At its core, ISCT Industry Membership provides the ability to network, discuss current challenges in Cell and Gene Therapy and examine potential solutions with global experts from a variety of disciplines. As part of the restructure, ISCT has adapted the membership program to two levels, Patron and Associate, both of which offer access to monthly strategic discussion forums, networking events, and the opportunity to contribute to the development of sector advancing educational resources.
The newly enhanced program also welcomed The Asia Pacific Regional Commercialization Committee, created to foster strong industry relationships in the region.
The launch of the new membership program marks a significant milestone for ISCT, with a record number of new members joining in 2022. ISCT Industry leadership looks forward to continuing to grow the ISCT global industry community and support those working to expand the development and delivery of safe and effective cell and gene therapies for patients.

Anthony Ting, PhD
BRL C> Consulting
United States
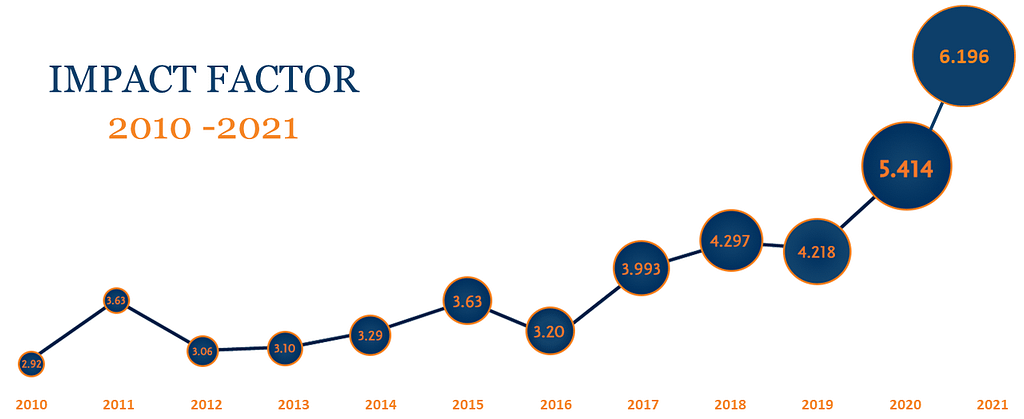
ISCT continues to advance scientific translation through the expansion and improvement of its peer-reviewed scientific journal, Cytotherapy. Under the close collaboration of its editorial board, with support from ISCT Head Office and our publishers at Elsevier, the Journal has attained a record-breaking impact factor at 6.196.
In 2022, Cytotherapy published 132 articles including, reviews, short repots, correspondence and editorials. The Journal received a record number of downloads and citations solidifying its position as the first choice in cell and gene therapy translation publishing.

Donald Phinney, PhD
Senior Editor
United States

Patrick Hanley, PhD
Commissioning Editor
United States
Take a look at the stunning submissions selected to be featured on the front cover of Cytotherapy® in 2022.
As a truly global organization, ISCT leverages a regional model to deploy solutions to global issues on a regional level. Spearheaded by passionate volunteer leadership, who understand the needs and concerns unique to each region, ISCT addresses the emergent needs of CGT professionals across the globe. With partnerships including regional partner organizations, regulatory bodies, and stakeholder communities, each of the ISCT regional committees drives the advancement of translational science at a high global standard.
Our committees work autonomously to deliver key scientific meetings, stimulate membership growth, and develop regional professional networks. Through 2022, our committees worked hard to develop the groundwork for new committees with a special focus on early-stage professionals and facilitated several major events that have continued to build consensus across the sector.
In 2022, to further the society’s commitment to workforce development, ISCT formed three new regional early-stage professionals subcommittees in Asia, Australia and New Zealand and South-Central America. Supported by the respective regional executive committee, these subcommittees were created to promote regional and global engagement and networking for early-stage professionals providing them with resources and career guidance to ensure their success.
Championed by regional volunteers, the Asia, Australia and New Zealand and South-Central America subcommittees are in full swing developing key initiatives to drive ESP membership and engagement in each region. ISCT is currently working on establishing ESP subcommittees in both North America and Europe for 2023.
ISCT regional committees deliver high-quality programmes globally, with autonomous planning supported by the society’s global network and resources. As restrictions eased post-pandemic, regional executive committees were excited to return to in-person meetings and reconnect face-to-face with the wider ISCT community.
With a reignited vigour to connect cell and gene therapy professionals globally, 2022 was a particularly active year for regional events. ISCT regional committees partnered and collaborated with several external groups and organizations as well connecting with other ISCT committees.
In August, the Australia & New Zealand Regional Executive Committee hosted its first in-person regional meeting post-pandemic. The highly anticipated event welcomed 137 delegates to Brisbane, Australia, and featured workshops and panel discussions with particular attention to quality, regulatory affairs, education, training, commercialization and clinical practice.
Meanwhile, in North America, the leadership team hosted a virtual interactive member-focused regional town hall meeting in October to connect and engage with members in the region. The meeting facilitated open discussions focused on workforce development and retention challenges, including both commercial and academic perspectives.
To facilitate cross-regional collaboration, The North America and South & Central America Regional Executive Committees partnered to present a two-part webinar series. The first installment discussed the clinical manufacturing of CAR T Cells, and the second explored the mechanism, safety and efficacy of mRNA-based cancer vaccines for oncological therapy. The collaborative series brought together subject matter experts and key opinion leaders from across both regions and received over 550 registrants from across the globe.


In Australia & New Zealand, ISCT leaders held a joint virtual scientific meeting with the Biotherapeutics Association of Australasia (BAA) to address developments in cell and gene therapies. The two-day event, held in February 2022, hosted scientific presentations featuring both international and regional speakers to discuss the latest developments in the clinical and commercial delivery of cell and gene therapies and the latest regulatory updates relevant to the region.
Leaders in Asia were delighted to continue its partnership with the Korean Society of Blood and Marrow Transplantation (KSBMT) with a joint session on gene editing and gene therapy at the International Congress of Blood and Marrow Transplantation (ICBMT), the 27th Annual congress of KSBMT in September. The long-standing partnership between ISCT and KSBMT is a key partnership for the region. It fosters international cooperation in education and research in the field of hematopoietic stem cell transplantation and cell & gene therapy.


In May, the ISCT Asia Regional Executive Committee partnered with the Japanese Society for Transplantation and Cellular Therapy (JSTCT 2022) to host a joint session on ‘Strategies to Improve Classical CBT by Introducing New Cellular Therapies’. The session was chaired by Satoshi Takahashi, MD, PhD, Past ISCT Asia Regional Vice-President, JSTCT 2022 President and included a remote presentation from William YK Hwang, MBBS, MMed, FRCP, FAMS, ISCT Asia Regional Vice-President Elect.
In October, the South and Central America Executive Committee again partnered with the Brazilian Association of Cellular and Gene Therapy (ABTCel-Gen) for the XII Congress of ABTCel – Gen in Rio de Janeiro. ISCT invited speakers participated both in-person and virtually, leading discussions on topics such as manuscript writing, cell therapy and therapies for cancer through sessions and interactive workshops. Alongside event participation, The South and Central America regional leaders were proud to sponsor the top-scoring abstract awards and provide a special abstract supplement showcasing all accepted abstracts from the meeting publication in Cytotherapy, the official journal of ISCT.




Janet Macpherson, PhD
Australia

C. Russell Y. Cruz, MD, PhD
USA
Available to ISCT members, Telegraft is the go-to resource to keep up to date with the latest Society activities and important developments in cell and gene therapy.
2022 brought exciting updates for Telegraft, with the launch of the ‘Telegraft Hub,’ the new online home for ISCT news and community. Alongside monthly e-newsletters, ISCT members now have access to the online interactive hub, allowing them to connect with peers and access key updates and curated content.
The Telegraft Editorial Board works to engage and connect the whole ISCT community across academia, science, regulation and commercial by sharing insightful discussions and up-to-date information on critical issues affecting the field of cellular therapy.

Michele Sugrue, MT
USA

Wouter Van’t Hof, PhD
USA

Joaquim Vives, PhD
Portugal

Rounak Dubey, MBBS, MD
India

Shyam Bhakta, MD, MBA
India

Ashley Krull, BSc, PhD
USA

Cytotherapy®, the official journal for the International Society for Cell and Gene Therapy (ISCT), publishes novel and innovative results from high quality scientific and clinical studies in the fields of cell and gene therapy. In 2021, Cytotherapy has continued to successfully advance and expand the field of cell and gene therapy for clinical researchers, oncologists, hematologists, physicians, and regulatory experts alike. The Journal had a 6.196 impact factor for 2022, a new record from which the editorial board hopes to grow further.
You can read issues of Cytotherapy® here.
Follow Cytotherapy® on Twitter to stay up to date on all the latest news and publications.
In January 2022, Dr. Rachel Burga was appointed as the new assistant editor for Cytotherapy. With a background in biomedical engineering and immunology, Rachel is the Principal Scientist of Cell Therapy at Obsidian Therapeutics. She has been an active member of ISCT since 2016 and currently sits on the Early-Stage Professionals Committee as Co-Chair.
As Assistant Editor, Rachel will work closely with Senior Editor Dr. Donald G. Phinney and Commissioning Editor, Dr. Patrick Hanley, to raise awareness of the Cytotherapy brand. In her first term, Rached helped recruit outstanding content for the journal and work closely with the ISCT membership to ensure that the journal continues to support the Society’s mission.

Rachel Burga, PhD
Assistant Editor, Cytotherapy
United States
Each year, ISCT hosts the Insta-Your-Cells Photo Challenge, calling on community members to submit interesting images from under the microscope. These images are selected to feature on the front cover of Cytotherapy®, the Official Journal of ISCT, showcasing the fascinating world of cells to peers around the globe.
The winning image of the 3rd annual Insta Your Cells Photo Challenge was submitted by Philippe Cohen, MD, PhD, France. ISCT Head Office prepared a framed keepsake to celebrate this iconic first-place image.
Take a look at the stunning submissions selected to be featured on the front cover of Cytotherapy® in 2022.


Emily Hopewell, PhD
Indiana University School Of Medicine
United States

Patrick Hanley, PhD
Children’s National Hospital
United States
Recognizing the current skills gaps in CGT translation as a critical issue for the sector, in November 2022, ISCT formed the ISCT Workforce Development Committee.
Led by Co-chairs Emily Hopewell, PhD and Patrick Hanley, PhD the committee will provide strategic guidance to identify, develop and deliver ISCT training and development programs.
Programs will focus on the specific needs of professions based on their region, industry, and skill level to support the sustainable growth of the cell and gene therapy sector.
Throughout 2023, the committee will focus on identifying and prioritizing urgent needs for training and educational programs for professionals working across the full spectrum of translational science, starting from pre-clinical development through to patient access, to develop and deliver comprehensive programs to upskill and train the existing CGT workforce.


Laertis Ikonomou, PhD
The State University of New York at Buffalo
United States
In October 2021, the ISCT Presidential Task Force on the Use of Unproven and/or Unethical Cell and Gene Therapies formally became the ISCT Committee on the Ethics of Cell and Gene Therapy (ECGT). This change is intended to reflect the committees revised mission to identify key ethical issues associated with the development, regulatory authorization, and distribution of cell and gene therapies.
In 2022, the Committee re-affirmed its position on, and concerns with, speculative immune cell banking. Following the 2022 US federal court ruling in favor of California Stem Cell Treatment Center, Inc., and Cell Surgical Network Corporation, the Committee contributed to publishing an ISCT position and press release, highlighting the dangers of unproven cell therapies to patient safety.
Undertaking its broader remit, the ECGT Committee developed and published informed positions providing guidance and clarity on complicated ethical matters within the CGT space. The Committee has undertaken the task of developing resources to address ethical topics, and potential issues, arising from the EU Hospital Exemption and the US expanded access pathway. The Committee organized a roundtable session at the ISCT 2022 San Francisco Annual Meeting addressing both pathways, and published a position paper on Hospital Exemption within the framework of safety and ethical interests of patients. On January 19, 2022, the Committee launched the ISCT Expanded Access Working Group.
Over the course of 2022, the Committee has worked to develop a guide for the general public, which analyses the regenerative medicine industry, focusing on distinctive features of unproven cell and cell-based products, suggesting practical strategies to address marketing of such products, and providing an overview of reporting mechanisms available to patients across different jurisdictions globally. This comprehensive guide will be published in 2023 and provides an important resource to protect patients globally.

Patricia J. Zettler, JD
The Ohio State University
United States

The ISCT Expanded Access Working Group was launched with the mandate to identify and address practical, ethical, and regulatory issues that arise from the use, and potential misuse, of the expanded access pathway by various CGT stakeholders.
Since its launch, the Working Group has been developing informed perspectives on expanded access to generate educational resources that promote innovation in translational research while upholding the highest ethical standards. The Working Group submitted a short report reviewing the history and current state of the US expanded access pathway, and highlighting three emerging areas of concern. The report will be published in Cytotherapy in early 2023.
The overarching objective of the Working Group is to further refine its position on ethical issues arising from the use, and potential misuse, of the expanded access pathway, culminating in the development of a code of ethics. In developing this code of ethics, ISCT will seek out input and endorsement from other organizations in the field.



Kevin Bosse, PhD RAC
Nationwide
Children’s Hospital
United States
Rachel Burga, PhD
Obsidian Therapeutics
United States
The ISCT Early Stage Professionals Committee (ESP) strives to engage and connect ESP with the wider CGT sector on a regional and global level. Co-Chaired by Kevin Bosse, PhD RAC and Rachel Burga, PhD, the committee provides networking opportunities and career development resources for professionals to excel in their careers.
The committee hosts a variety of tailored events and workshops, such as the ISCT ESP Mentoring Program and the ISCT ESP Leadership Development program helping connect professionals with key opinion leaders and subject matter experts.
In 2022, ISCT formed three regional ESP subcommittees in Asia, Australia and New Zealand and South Central America to drive ESP membership and increase interactions between ESPs and regional leadership.

Cytotherapy®, the official journal for the International Society for Cell and Gene Therapy (ISCT), publishes novel and innovative results from high quality scientific and clinical studies in the fields of cell and gene therapy.In 2021, Cytotherapy has continued to successfully advance and expand the field of cell and gene therapy for clinical researchers, oncologists, hematologists, physicians, and regulatory experts alike. The Journal had a 5.414 impact factor for 2020, a new record from which the editorial board hopes to grow further.
This year, ABTCel-Gen, a CGT-focused society based in South & Central America, joined as an affiliate society to Cytotherapy. This landmark partnership creates a strong pathway to publication for affiliate members, while drawing from a proven pool of translational expertise.
Take a look at our Talking with Giants series, launched in 2019, which showcases leaders in the field of cell and gene therapy through informal question and answer articles. Talking with Giants is featured at the start of each issue of Cytotherapy.
You can read issues of Cytotherapy® here.
This year, Cytotherapy® also launched a Twitter account to share fresh updates and resources.
Each year, ISCT hosts the Insta-Your-Cells Photo Challenge, calling on community members to submit interesting images from under the microscope. These images are selected to feature on the front cover of Cytotherapy®, the Official Journal of ISCT, showcasing the fascinating world of cells to peers around the globe.
This year, ISCT Head Office also prepared a framed keepsake to celebrate an iconic first place image, submitted by Pradyut Paul, PhD, University of Wisconsin-Madison, United States.

Take a look at the stunning submissions selected to be featured on the front cover of Cytotherapy® this year.



Janet Macpherson, PhD
Australia

C. Russell Y. Cruz, MD, PhD
USA
Available to ISCT members, Telegraft is the go-to resource to keep up to date with the latest Society activities and important developments in cell and gene therapy.
2022 brought exciting updates for Telegraft, with the launch of the ‘Telegraft Hub,’ the new online home for ISCT news and community. Alongside monthly e-newsletters, ISCT members now have access to the online interactive hub, allowing them to connect with peers and access key updates and curated content.
The Telegraft Editorial Board works to engage and connect the whole ISCT community across academia, science, regulation and commercial by sharing insightful discussions and up-to-date information on critical issues affecting the field of cellular therapy.

Nancy H. Collins, PhD
USA

Satyam Arora, MD
USA

Rounak Dubey, MBBS, MD
India

Shyam Bhakta, MD, MBA
India

Ashley Krull, BSc, PhD
USA

Michele Sugrue, MT
USA

Wouter Van’t Hof, PhD
USA

Joaquim Vives, PhD
Portugal
ISCT acknowledges the service of its members, volunteering expertise, time, and energy to the vast task of advancing the field of cell and gene therapies.
From mentoring the next generation to publishing key standards and fostering relationships across the sector, ISCT members set the gold standard.
We acknowledge these Society leaders who have completed terms of service in a formal position in 2021.
Your hard work and dedication have continually pushed the society to new heights, and we cannot wait to connect again soon:

John Rasko, AO, MBBS, PhD, FRCPA, FRACP, FAHMS
Chair, Strategic Advisory Council (Immediate Past President)
June 2020 – June 2022
Royal Prince Alfred Hospital
Sydney, Australia

Lizette Caballero, BS, MT(ASCP)
Global Secretary
June 2019 – June 2022
UCSF Blood and Marrow Transplant Lab
San Francisco, CA, United States

Rajiv Khanna, PhD AO
Australia & New Zealand, Regional Vice-President
June 2020 – June 2022
QIMR Berghofer Medical Research Institute
Brisbane, Australia

Mitchell Cairo, MD
North America, Regional Vice-President
June 2020 – June 2022
New York Medical College
New York, NY, United States

Karen Nichols, Esq.
Chief Regulatory Officer
March 2016 – June 2022
Vertex Pharmaceuticals
Cambridge, MA, United States

Vicki Antonenas, BSc, MSc
Elected Member Technologist
June 2020 – June 2022
Sydney Cellular Therapies Laboratory,
Westmead Hospital
Australia

Patrick Hanley, PhD
Co-Chair Immuno-Gene
Therapy Committee
Children’s National Hospital
United States

Giuseppe Orlando, MD, PhD
Co-Chair Gastrointestinal Committee
Wake Forest University
United States

Rachele Ciccocioppo, MD
Co-Chair Gastrointestinal Committee
University of Verona
Italy

George Muschler, MD
Co-Chair Orthopedic & Musculoskeletal Therapies
Cleveland Clinic
United States

Christian Jorgensen, MD, PhD
Co-Chair Orthopedic & Musculoskeletal Therapies
University Hospital Lapeyronie
France

Sarah Chan, Ma PhD
ECGT Committee
University of Edinburgh
United Kingdom

Andrew Hoffman, DVM, DVSc
Exosomes Committee
University of Pennsylvania
United States

John Fink, MBA
Co-Chair Process Development and Manufacturing Committee
PerkinElmer, Inc.
United States

Emily Hopewell, PhD
Indiana University School of Medicine
United States

Aaron Tze Kai Tan
Stanford University
United States

Lizbeth Diaz Polo, MD
University of San Martin de Porres
Spain

Jordan Greenberg, PhD
Shoreline Biosciences
United States

Shabnum Patel, PhD
CARGO Therapeutics
United States

Nancy H. Collins, PhD
University of Toledo
Toledo, OH, United States

Satyam Arora, MD
Super Speciality Paediatric Hospital and Post Graduate Teaching Institute
India

Lab Practices Committee
Vicki Antonenas, BSc, MSc
Westmead Hospital
Australia

Europe Legal & Regulatory Affairs Committee
Paula Salmikangas, PhD
NDA Group
Finland
As a truly global organization, ISCT leverages a regional model to deploy solutions to global issues on a regional level. Spearheaded by passionate volunteer leadership, who understand the needs and concerns unique to each region, ISCT addresses the emergent needs of CGT professionals across the globe. With partnerships including regional partner organizations, regulatory bodies, and stakeholder communities, each of the ISCT regional committees drives the advancement of translational science at a high global standard.
Our committees work autonomously to deliver key scientific meetings, stimulate membership growth, and develop regional professional networks. Through 2022, our committees worked hard to develop the groundwork for new committees with a special focus on early-stage professionals and facilitated several major events that have continued to build consensus across the sector.
In 2022, to further the society’s commitment to workforce development, ISCT formed three new regional early-stage professionals subcommittees in Asia, Australia and New Zealand and South-Central America. Supported by the respective regional executive committee, these subcommittees were created to promote regional and global engagement and networking for early-stage professionals providing them with resources and career guidance to ensure their success.
Championed by regional volunteers, the Asia, Australia and New Zealand and South-Central America subcommittees are in full swing developing key initiatives to drive ESP membership and engagement in each region. ISCT is currently working on establishing ESP subcommittees in both North America and Europe for 2023.
ISCT regional committees deliver high-quality programmes globally, with autonomous planning supported by the society’s global network and resources. . As restrictions eased post-pandemic, regional executive committees were excited to return to in-person meetings and reconnect face-to-face with the wider ISCT community.
With a reignited vigour to connect cell and gene therapy professionals globally, 2022 was a particularly active year for regional events. ISCT regional committees partnered and collaborated with several external groups and organizations as well connecting with other ISCT committees.
In August, the Australia & New Zealand Regional Executive Committee hosted its first in-person regional meeting post-pandemic. The highly anticipated event welcomed 137 delegates to Brisbane, Australia, and featured workshops and panel discussions with particular attention to quality, regulatory affairs, education, training, commercialization and clinical practice.
Meanwhile, in North America, the leadership team hosted a virtual interactive member-focused regional town hall meeting in October to connect and engage with members in the region. The meeting facilitated open discussions focused on workforce development and retention challenges, including both commercial and academic perspectives.
To facilitate cross-regional collaboration, The North America and South & Central America Regional Executive Committees partnered to present a two-part webinar series. The first installment discussed the clinical manufacturing of CAR T Cells, and the second explored the mechanism, safety and efficacy of mRNA-based cancer vaccines for oncological therapy. The collaborative series brought together subject matter experts and key opinion leaders from across both regions and received over 550 registrants from across the globe.


In Australia & New Zealand, ISCT leaders held a joint virtual scientific meeting with the Biotherapeutics Association of Australasia (BAA) to address developments in cell and gene therapies. The two-day event, held in February 2022, hosted scientific presentations featuring both international and regional speakers to discuss the latest developments in the clinical and commercial delivery of cell and gene therapies and the latest regulatory updates relevant to the region.
Leaders in Asia were delighted to continue its partnership with the Korean Society of Blood and Marrow Transplantation (KSBMT) with a joint session on gene editing and gene therapy at the International Congress of Blood and Marrow Transplantation (ICBMT), the 27th Annual congress of KSBMT in September. The long-standing partnership between ISCT and KSBMT is a key partnership for the region. It fosters international cooperation in education and research in the field of hematopoietic stem cell transplantation and cell & gene therapy.


In May, the ISCT Asia Regional Executive Committee partnered with the Japanese Society for Transplantation and Cellular Therapy (JSTCT 2022) to host a joint session on ‘Strategies to Improve Classical CBT by Introducing New Cellular Therapies’. The session was chaired by Satoshi Takahashi, MD, PhD, Past ISCT Asia Regional Vice-President, JSTCT 2022 President and included a remote presentation from William YK Hwang, MBBS, MMed, FRCP, FAMS, ISCT Asia Regional Vice-President Elect.
In October, the South and Central America Executive Committee again partnered with the Brazilian Association of Cellular and Gene Therapy (ABTCel-Gen) for the XII Congress of ABTCel – Gen in Rio de Janeiro. ISCT invited speakers participated both in-person and virtually, leading discussions on topics such as manuscript writing, cell therapy and therapies for cancer through sessions and interactive workshops. Alongside event participation, The South and Central America regional leaders were proud to sponsor the top-scoring abstract awards and provide a special abstract supplement showcasing all accepted abstracts from the meeting publication in Cytotherapy, the official journal of ISCT.

Building Consensus and Community
Motivated to stay true to the essence of ISCT as a truly global translation-focused community of experts, ISCT made major strategic progress in 2022, with significant investments in community development, workforce development and addressing ethical issues. Working in collaboration with ISCT leadership, the Society enhanced the value of its membership value across all disciplines for leaders and newcomers alike as well as developed key resources and drove consensus to combat the critical issues facing the wider CGT sector. As the ISCT community continues to grow, the Society is perfectly positioned to empower the field to drive the translation of research into safe, effective and accessible treatments with long-lasting impact.

Excited to welcome delegates to the first in-person ISCT meeting post Pandemic, ISCT was determined to make a significant impact with ISCT 2022 San Francisco. Welcoming 1,646 delegates from across the globe, ISCT 2022 was the largest ISCT Annual Meeting to date and was proud to serve as a key event reconnecting the wider CGT community. A standout feature of the meeting was the redesign of the ISCT Scientific program, a strategic initiative inspired to better cater to the interests of all delegates, across the full range of CGT development and deployment.
The Translational Pathway Program, reflects ISCT’s role in driving the translation of scientific research into safe and effective therapies that can be deployed to patients. The new program was designed to mirror the therapeutic development process and integrated perspectives across the CGT sector to address key topics at all stages of translation.
As a counter-part to the Translational Pathway Program, ISCT also launched the Roundtable Program at ISCT 2022. Unique in the cell and gene therapy sector, this program provides a platform for delegates to debate and collaboratively develop consensus and solutions to practical problems and barriers in the field. Recognizing the breadth and depth in expertise of Annual Meeting delegates, ISCT developed this unique platform to connect professionals from across the globe who share the same goals and encounter the same difficulties.
“ISCT broadened its focus, through a whole series of roundtables, to cover wider challenges experienced across the industry, from generating return on investment to managing the supply chain. ISCT will continue to monitor the efficacy of solutions generated at the meeting and continue to work with all stakeholders to ensure an increasing number of patients are able to benefit from cell and gene therapies.”

Bruce Levine, PhD
University Of Pennsylvania
United States
The rapid growth of the sector has introduced new ethical concerns associated with the development, regulatory authorization, and distribution of cell and gene therapies. Recognizing this, ISCT has worked to develop internal infrastructure so that the Society remains a leading voice on critical and topical ethical issues.
Undertaking a broader remit to identify these key ethical issues, ISCT has introduced the Committee on the Ethics of Cell and Gene Therapy (ECGT). Formally the ISCT Presidential Task Force on the Use of Unproven and/or Unethical Cell and Gene Therapies, this new multidisciplinary committee regroups key stakeholder perspectives to promote the ethical development of the sector.
To further refine the Society’s position on ethical issues, ISCT formed the ISCT Expanded Access Working Group. The overarching objective of the Working Group is to further refine its position on ethical issues arising from the use, and potential misuse, of the expanded access pathway, culminating in the development of a code of ethics.
Under the guidance of the new ECGT committee, ISCT is now better positioned to give informed consensus on ethical issues. In 2022, following the 2022 US federal court ruling in favor of California Stem Cell Treatment Center, Inc., and Cell Surgical Network Corporation, ISCT published a press release highlighting the dangers of unproven cell therapies to patient safety.
With Industry members representing a vital part of ISCT DNA, in 2022, ISCT worked to adapt the industry program to better meet the needs of our growing community. In collaboration with the ISCT Industry leadership, ISCT worked to evaluate how to adapt our program gaining feedback from our members at the forefront of driving the translation of cell and gene therapies.
At its core, ISCT Industry Membership provides the ability to network, discuss current challenges in Cell and Gene Therapy and examine potential solutions with global experts from a variety of disciplines. As part of the restructure, ISCT has adapted the membership program to two levels, Patron and Associate, both of which offer access to networking events, monthly strategic discussion forums and the opportunity to contribute to the development of sector advancing educational resources.
The newly enhanced program also welcomed The Asia Pacific Regional Commercialization Committee, created to foster strong industry relationships in the region.
The launch of the new membership program marks a significant milestone for ISCT, with a record number of new members joining in 2022. ISCT Industry leadership looks forward to continuing to grow our global community and support those working to expand the development and delivery of safe and effective cell and gene therapies for patients.

Anthony Ting, PhD
Bone Therapeutics
United States

ISCT continues to advance scientific translation through the expansion and improvement of its peer-reviewed scientific journal, Cytotherapy. Under the close collaboration of its editorial board, with support from ISCT Head Office and our publishers at Elsevier, the Journal has attained a record-breaking impact factor at 6.196.
In 2022, Cytotherapy published 132 articles including, reviews, short repots, correspondence and editorials. The Journal received a record number of downloads and citations solidifying its position as the first choice in cell and gene therapy translation publishing.

Donald Phinney, PhD
Senior Editor
United States

Patrick Hanley, PhD
Commissioning Editor
United States

Take a look at the stunning submissions selected to be featured on the front cover of Cytotherapy® in 2022.

Bruce Levine, PhD
President
June 2020 – June 2022
Barbara and Edward Netter Professor in Cancer Gene Therapy
University of Pennsylvania
Philadelphia, PA, United States

Jacques Galipeau, MD
President-Elect
June 2020 – June 2022
University of Wisconsin-Madison
Madison, WI, United States

John Rasko, AO, MBBS, PhD, FRCPA, FRACP, FAHMS
Chair, Strategic Advisory Council (Immediate Past President)
June 2020 – June 2022
Royal Prince Alfred Hospital
Sydney, Australia

Lizette Caballero, BS, MT(ASCP)
Global Secretary
June 2019 – June 2022
UCSF Blood and Marrow Transplant Lab
San Francisco, CA, United States

Emily Hopewell, PhD
Global Treasurer
June 2020 – June 2023
Indiana University School of Medicine
Zionsville, Indiana

Bryan Choi, PhD
Asia, Regional Vice-President
June 2021 – June 2023
Strategic Center For Regenerative Medicine (SCRM)
Inha University
Incheon, Korea


Massimiliano Gnecchi, MD, PhD
Europe, Regional Vice-President
June 2021 – June 2023
University Of Pavia & IRCCS Policlinico San Matteo Hospital
Pavia, Italy

Mitchell Cairo, MD
North America, Regional Vice-President
June 2020 – June 2022
New York Medical College
New York, NY, United States

Antonio Carlos Campos de Carvalho, MD, PhDSouth and Central America, Regional Vice-President
June 2021 – June 2023
Institute Of Biophysics
Federal University Of Rio De Janeiro
Rio De Janeiro, Brazil

Daniel J. Weiss, MD, PhD
Chief Scientific Officer
June 2016 – June 2022
University of Vermont
Burlington, VT, United States

Anthony Ting, PhD
Chief Commercialization Officer
May 2020 – May 2022
Athersys
Cleveland, OH, United States

Karen Nichols, Esq.
Chief Regulatory Officer
March 2016 – June 2022
Vertex Pharmaceuticals
Cambridge, MA, United States

Dominique Farge, MD, PhD
Elected Member MD
June 2021 – June 2023
Greater Paris University Hospitals – AP-HP
Paris, France

Sowmya Viswanathan, PhD
Elected Member PhD
June 2020 – June 2022
University Health Network and University of Toronto
Canada

Vicki Antonenas, BSc, MSc
Elected Member Technologist
June 2020 – June 2022
Sydney Cellular Therapies Laboratory,
Westmead Hospital
Australia

Diane Kadidlo, BSc, MT(ASCP), SBB
Elected Member Technologist
June 2021 – June 2023
Molecular And Cellular Therapeutics
University Of Minnesota
St Paul, Minnesota, United States

Donald Phinney, PhD
Senior Editor of the Journal
October 2018 – Present
Professor at The Scripps Research Institute
Jupiter, FL, United States

Queenie Jang, BSc (Pharmacy), MBA
Chief Executive Officer
ISCT
Vancouver, BC, Canada


