Adoptive cell therapy of tumor-infiltrating lymphocytes has shown promise for treatment of refractory melanoma and other solid malignancies; however, challenges to manufacturing have limited its widespread use. Traditional manufacturing efforts were lengthy, cumbersome and used open culture systems. We describe changes in testing and manufacturing that decreased the process cycle time, enhanced the robustness of critical quality attribute testing and facilitated a functionally closed system. These changes have enabled export of the manufacturing process to support multi-center clinical trials.
If you are seeing this, please disable your adblock software to view the full version of our annual report!
Cultivating Our Roots
ISCT Board of Directors, Strategic Planning 2020-2022 Event. Vancouver, Canada. February 2019
Cultivating Our Roots
ISCT Board of Directors, Strategic Planning 2020-2022 Event. Vancouver, Canada. February 2019

John EJ Rasko, AO, MBBS, PhD,
FRCPA, FRACP, FAHMS
ISCT President (2018-2020)
Australia
These Are Challenges That ISCT Was Made For
The science is advancing, but the workforce needed to make it into an accessible clinical reality is not yet here.
I am genuinely excited to welcome you to ISCT’s second annual report. This cornerstone publication provides an overview of our strategic trajectory, and a moment of appreciation and acknowledgment for the contributions of our membership across the world.
Last year, we saw the start of a golden age for cell and gene therapy, and the accelerated evolution of our society. This year, we are taking steps to further sustain our growth.
Our Society has evolved in the past few years to meet the ambitions of a golden age of cell and gene therapy. Alongside that ambition, it is important to acknowledge where we have come from. The current state of cell and gene therapy is the result of decades of hard work, passionate dedication, and tireless perseverance.
From our pioneering investigators and lab technologists, to our regulatory stewards, to our business and industrial leaders, ISCT maintains a continued and comprehensive trajectory of advancement for our field. This is an opportune time to look back and to reflect upon our roots to cultivate the developments that we will enable and indeed push into the future.
Within this report, you will see our efforts over the past year to connect membership across our global Society, to communicate to the public responsibly and respectfully, and to translate cutting edge scientific inquiry into concrete clinical procedures and products. In short, we are here after more than a quarter of a century to continue our mission to connect, communicate and translate.
This year, we are introducing a “Strategic Features” section, where you will learn about key ISCT initiatives. Here, you will have a chance to learn about our efforts to engage lab technologists and the dramatic impact of our mentorship program. You will also learn more about the successes of our Cell Therapy Training Course, updates to our global engagement model, and the exciting reshaping of Cytotherapy, our flagship publication.
In helping the public access safe and effective cell and gene therapies through our Presidential Task Force on the Use of Unproven and/or Unethical Cell and Gene Therapies; in serving together across fields of academia, regulation, and commercialization to improve lives around the world, we have amassed a wealth of allies working to a common goal that has expanded substantially over the past year.
The past year also marks the growth of a sustained commitment and growing pursuit of mentorship within our Society. With our mentorship program at its third year, we have achieved exciting and unprecedented growth and passionate interest driving engagement at a global level. Our mentorship program focuses on career and professional development, but it is also built for members to connect personally and to develop collaborative dialogue that works to continually advance our field.
My term as President of the Society has been characterized by a commitment to the advancement of our communications. From our Board summit that brought our leadership team together in Vancouver in January 2019, to our annual and regional meetings which connect members from across the globe, we have prioritized personal engagement as part of our communicative strategy. We have reached out to allied organizations to create agreements and strategic partnerships, including significant memoranda of understanding laying out concrete action plans for the future we share with our allies. The Signature Series of our Scientific and Commercialization Committees are just one strong example that successfully showcases what we bring to the table.
At its core, ISCT is built on the fundamental ideal of connection. This key aspect of our identity leads me to believe that ISCT represents a guiding beacon for the tsunami of innovation that is cell and gene therapy today.
Over the past year, blood stem cell gene therapy for transfusion-dependent thalassemia was approved in Europe. Gene-directed immunotherapy techniques have undergone significant consolidation across the globe. Reimbursement and clinical implementation issues are at the forefront of focus for governments and industry. The science is advancing, but the workforce needed to make it into an accessible clinical reality is not yet here.
Ahead of us lie great challenges if we are to sustain our growth and meet emerging demands in our field. The Good Ship “Cell and Gene Therapy” needs more hands on deck! Laboratories need to be supported as they scale upwards to meet industrial requirements. Training must be expanded so that expertise can grow and spread.
These are challenges that ISCT was made for. The pioneers and leaders we honour in our field have set strong foundations and provided a vision for a world where those with unmet medical needs have hope.
They point to a bright future ahead of us. As we continue to carry the momentum of decades into the work ahead, I invite you to rejoice with me at the flourishing of our field. Let us continue to cultivate our roots, and do the good work now needed to advance onwards.

Queenie Jang, BSc (Pharmacy), MBA,
Chief Executive Officer
Canada
There Is No Society Like Ours
ISCT is unique. Because of this, we stand ready to advance our mission and to also lead others with this pursuit.
Our Society has grown throughout 2019.
This is a fact that characterizes the second annual report of the ISCT. Throughout the year, we have worked to rekindle our roots. Not only did we renew our focus towards CGT labs across the globe, but we also worked to foster an environment of mentorship within the Society. Along the way, we expanded our global footprint by making deeper connections across our regional partnerships.
Following up on a year of evolution through these actions, we are pushing the growth of the society towards a sustainable direction.
The passion across our leadership and membership has been palpable this year. The guidance of our ESP committee has resulted in an inspiring mentorship engagement. The new vision of Cytotherapy engages technologists and investigators alike. Meanwhile, the increasing success of our annual and regional meetings points to one realization:
ISCT is ready to take on the challenges that lie ahead for the cell and gene therapy field.
Throughout 2019, the legacy of the ISCT shone bright.
Key contributors like co-founder Dr. Nancy Collins returned to help push the Society forwards. A renewed partnership with our daughter organization FACT helped us engage technologists globally. All the while, the insights of our founders have paid massive dividends towards our task today.
The founding vision of ISHAGE, which would become the ISCT, identified key needs that have now grown urgent. The field needs to develop a robust workforce. It needs programs that kickstart sustainable cycles of knowledge growth. It also needs collaborative communication platforms that emphasize a global membership. The efforts of our founders now give us momentum to push these key areas.
We have taken this momentum and pushed to engage labs on a global scale. Across academia and industry, ISCT provides useful resources and communications infrastructure. We support technologists developing manufacturing and quality control procedures necessary for clinical advancement.
We have pushed to cultivate scientific expertise around cell and gene therapy across the globe. Our ISCT Mentorship and Cell Therapy Training Course programs provide a platform for scientific leaders across the globe to cultivate the next generation of investigators.
We have pushed, finally, to engage the scientific community through Telegraft and Cytotherapy. Both publications have adopted methods to more deeply engage with collaborators. The editorial boards across both publications have also recruited to expand their expertise in CGT. In 2019, both publications took on vibrant visual redesigns and refining the scope of their visions.
This is an exciting moment where the potential of our field demands decisive action.
As the science races forward, cell and gene therapy requires people that can implement it. We must continue to cultivate technologists in laboratories, effective public-facing communicators, conscientious accreditors, and ongoing clear-sighted leadership. After all, there is no other Society like us.
We exist at a global scale. We engage the entire translational chain of science behind cell and gene therapy. We share a common vision, carried forward by over 41 standing committees.
ISCT is unique. Because of this, we stand ready to advance our mission and to also lead others with this pursuit.
With us are an effective and global network of collaborators, ranging from state regulatory bodies to industrial powerhouses to key academic opinion leaders. Last year, we drew on connections across this network to hold joint meetings across the world. These include SCSS-ISCT in the Asia region, ISCT ANZ-ISSCR-ASGCT in the Australia and New Zealand region, and our ISCT-SCA regional meeting under the umbrella of SOMICET’s Scientific Congress in the South and Central America region. We have developed joint initiatives that will sustain the advancement of the field so that we can address the gap between what we know and what we can implement.
The Society continues to advance. As we enter 2020, we will continue to optimize our engagement platforms for members. We will continue to grow our membership globally. We will work together to draw the future of the field into present realities, and by doing so, move from rekindling our roots to defining our legacy.
Our Board of Directors (2019-2020)
The ISCT Board of Directors consists of:
- President (Chair of the Board of Directors)
- President-Elect
- Immediate Past President (Chair of the Strategic Advisory Council)
- Global Secretary
- Global Treasurer
- Regional Vice Presidents
- Chief Scientific Officer
- Chief Regulatory Officer
- Chief Commercialization Officer
- 4 Elected ISCT Members
- Senior Editor of the Society’s Journal
- Chief Executive Officer.
The Board of Directors is the main administrative body which manages the governance and strategic oversight of the Society.
Chair

John Rasko, AO, MBBS, PhD, FRCPA, FRACP, FAHMS
President
June 2018 – June 2020
Royal Prince Alfred Hospital
Sydney, Australia
Directors

Bruce Levine, PhD
President-Elect
June 2018 – June 2020
Barbara and Edward Netter Professor in Cancer Gene Therapy
University of Pennsylvania
Philadelphia, PA, United States

Catherine Bollard, MBChB, MD
Chair, Strategic Advisory Council (Immediate Past President)
June 2018 – June 2020
Children’s National Health System
George Washington University
Washington, DC, United States

Lizette Caballero, BS, MT(ASCP)
Global Secretary
June 2019 – June 2022
UCSF Blood and Marrow Transplant Lab
San Francisco, CA, United States

Emily Hopewell, PhD
Interim Global Treasurer
June 2019 – June 2020
Indiana University School of Medicine
Zionsville, Indiana

Oscar Lee, MD, PhD
Asia, Regional Vice-President
June 2019 – June 2021
National Yang- Ming University
Taiwan

Ngaire Elwood, PhD
Australia & New Zealand, Regional Vice-President
June 2018 – June 2020
Murdoch Childrens Research Institute
Melbourne, Australia

Joan Garcia-Lopez, MD, PhD
Europe, Regional Vice-President
June 2019 – June 2021
Director of Research and Education Banc De Sang i Teixits Barcelona, Spain

Lynn O’Donnell, PhD
North America, Regional Vice-President
June 2018 – June 2020
Ohio State University, James Cancer Hospital
Columbus, OH, United States

Patricia Rocco, MD, PhD
South and Central America, Regional Vice-President
June 2019 – June 2021
Federal University of Rio de Janeiro
Rio de Janeiro, Brazil

Daniel J. Weiss, MD, PhD
Chief Scientific Officer
June 2016 – June 2020
University of Vermont
Burlington, VT, United States

Miguel Forte, MD, PhD
Chief Commercialization Officer
June 2016 – June 2020
Zelluna Immunotherapy
Oslo, Norway

Karen Nichols, Esq.
Chief Regulatory Officer
March 2016 – March 2019
Magenta Therapeutics
Cambridge, MA, United States

Elizabeth Stenger, MD, MSc
Elected Member MD
June 2019 – June 2021
UPMC Children’s Hospital of Pittsburgh
Pittsburgh, PA, United States

Nadim Mahmud, MBBS, PhD
Elected Member PhD
June 2018 – June 2020
University of Illinois College of Medicine
Chicago, IL, United States

Jeannette Bloom, MBA, MT(ASCP)SBB
Elected Member Technologist
June 2018 – June 2020
Baylor College of Medicine
Houston, TX, United States

Anne Lamontagne, MSc
Elected Member Technologist
June 2019 – June 2021
Clinical Cell and Vaccine Production Facility
University of Pennsylvania
Philadelphia, PA, United States

Donald Phinney, PhD
Senior Editor of the Journal
October 2018 – Present
Professor at The Scripps Research Institute
Jupiter, FL, United States

Queenie Jang, BSc (Pharmacy), MBA
Chief Executive Officer
ISCT
Vancouver, BC, Canada
Our Executive Management Committee
The ISCT Executive Management Committee consists of:
- Chief Executive Officer (Chair of the Executive Management Committee),
- President
- President-Elect
- Immediate Past President (Chair of the Strategic Advisory Council)
- Global Secretary
- Global Treasurer
The Executive Management Committee oversees the operational and management aspects of the Society.
Chair

Queenie Jang, BSc (Pharmacy), MBA
Chief Executive Officer
ISCT
Vancouver, BC, Canada
Members

John Rasko, AO, MBBS, PhD, FRCPA, FRACP, FAHMS
President
June 2018 – June 2020
Royal Prince Alfred Hospital
Sydney, Australia

Bruce Levine, PhD
President-Elect
June 2018 – June 2020
Barbara and Edward Netter Professor in Cancer Gene Therapy
University of Pennsylvania
Philadelphia, PA, United States

Catherine Bollard, MBChB, MD
Chair, Strategic Advisory Council (Immediate Past President)
June 2018 – June 2020
Children’s National Health System
George Washington University
Washington, DC, United States

Lizette Caballero, BS, MT(ASCP)
Global Secretary
June 2019 – June 2022
UCSF Blood and Marrow Transplant Lab
San Francisco, CA, United States

Emily Hopewell, PhD
Interim Global Treasurer
June 2019 – June 2020
Indiana University School of Medicine
Zionsville, Indiana
A Renewed Focus on Laboratories and Technologists
Developed By ISCT Members, For ISCT Members
The ISCT Laboratory Life Line
In 2019, our Lab Practices Committee founded the ISCT Laboratory Life Line, taking a proactive and leading step towards promoting high standards and knowledgeable protocols across our membership in lab settings. This forum is designed as an interactive resource where professionals can engage in shop talk, facilitating collaboration, troubleshooting, and engagement for lab personnel. We encourage interested membership to contribute and learn about best practices for lab practices, leadership, or organization through accessible online discussions at our Life Line.
Our community engagement is the cornerstone of this effort
In today’s ever-changing landscape of cell and gene therapy, it is more important than ever to capitalize on the collective expertise of ISCT members to drive best practices. The Laboratory Life Line is one platform that was developed to encourage lab professionals of all backgrounds to share ideas, collaborate and educate one another to promote excellence across the cell therapy field.
Our community engagement is the cornerstone of this effort.

Heather Garrity, BSc, MSc
Co-Chair, ISCT Lab Practices Committee
United States
ISCT-FACT Partnership
We began a new partnership over the past year with one of our closest collaborators, the Foundation for Accreditation of Cellular Therapy (FACT), with the goal of spreading educational resources across the membership embedded within a wide network of laboratories. The start of this partnership was exciting not only for the tangible benefits that we worked together to bring to a wider network, but also because through it, we worked to rekindle our roots through the shared heritage of our organizations.

Nancy Collins, PhD
Co-Founder, ISCT
Founding Board Member, FACT
United States
An unprecedented journey of communication, education, and trust between practitioners and regulators
The primary motivators for founding ISHAGE (now ISCT) and ASBMT (now ASTCT), was concern for development of hematopoietic stem cell transplantation, patient safety protection, and translation of cutting edge research into clinical application. In the early years, we were well aware that the FDA feared unsafe development in our field, and we feared adoption of a drug development regulatory model that did not fit cell therapy.
ISHAGE and ASBMT leaders founded FACT in 1996 as a voluntary inspection and accreditation organization to be a single voice for collection, processing, and clinical centers which used the best and safest practices. The resulting FACT and FDA collaboration has been an unprecedented journey of communication, education, and trust between practitioners and regulators. It has fundamentally changed both groups, resulting in regulations and guidances which are informed by FACT accredited organizations. This model is even more important today since the expansion of hematopoietic transplantation to include all cell types in the new field of regenerative medicine,
More than ever, it is important that all laboratories have access to educational resources and have tools to educate and train the next generation of cell and gene therapy professionals.
Medical laboratory professionals play a vital role in cellular therapy. Even with the best quality management systems in place, quality results are difficult to ensure without adequately trained staff.
Personnel at FACT-accredited labs are required to be knowledgeable about FACT Standards and federal regulations. An ISCT Laboratory Membership can provide technologists access to ISCT-sponsored meetings and ongoing online educational resources aimed at providing solutions for quality, operational, and regulatory challenges in the Cell and Gene Therapy Lab at a discounted rate.
FACT has partnered with ISCT to provide a special Laboratory Membership offer to FACT-accredited laboratories. This offer includes 3 complimentary technologist memberships with a 2020 ISCT Lab Membership. To ensure all staff have access to ISCT resources including education, forums, the Telegraft, and Cytotherapy, additional technologist memberships are highly subsidized!

Linda Miller
Chief Executive Officer, FACT
United States
Engaging Industry Labs
As we work to advance the field of Cell and Gene Therapy, labs dedicated to industrial manufacturing have been on the forefront of our strategic vision. Since our last annual report, we worked to engage laboratories working under the umbrella of our industry partners, to assist them in identifying key personnel requirements, in recruiting highly qualified personnel to meet these requirements, and to train technicians where there are gaps in the pool of available applicants.

Gerhard Bauer, PhD
Laboratory Director, GMP Facility, UC Davis
United States
They don’t need master’s degrees; they need to receive appropriate manufacturing training and learn how to work within a GMP environment
Good Manufacturing Process (GMP) manufacturing of approved, commercialized cell and gene therapy products, and late phase clinical trial products slated for the market, has already become a major part of our industry.
Over the last three years we have been able to produce overwhelming evidence that such products can save lives of patients who did not have a great chance of survival only a few years earlier. We do face, however, a challenge in bringing a sufficient quantity of such products to market while keeping the price within an affordable range. We therefore need to find better ways of manufacturing cell and gene therapy products.
For this purpose, what is still needed is a larger work force of cell and gene therapy hands on product manufacturers; they don’t need master’s degrees; they need to receive appropriate manufacturing training and to learn how to work within a GMP environment, faithfully executing tasks under supervision proficient in Quality Control and Quality Assurance. If we want to get more life-saving products to patients, then we need to develop educational programs for such qualified personnel, and will need to coordinate our efforts so students can have access to such programs at several centers of excellence.
An Allied Perspective – CCRM’s Strategy to Support Commercialization
CCRM is accelerating the translation of promising technologies, processes and therapies into life-changing health outcomes for patients.
We are making this possible by providing strategic funding, dedicated infrastructure and specialized business and scientific expertise to support the commercialization of cell and gene therapies. We have partnered with leading research institutions to launch new ventures, and we enable industry by providing innovative contract development manufacturing organization (CDMO) services and scaling emerging companies by catalyzing investment.
Supporting and enabling training, to ensure we have a skilled workforce, is another area we are tackling. The industry has many challenges to overcome and we have made it our purpose to solve the big problems in regenerative medicine.

Michael May
Chief Executive Officer, CCRM
Canada
ESP Mentorship as a Model for Global Expertise
A Mentor’s Perspective
Diseases know no borders. Global problems need global and new solutions. Developing innovative therapies requires the involvement of committed individuals whose talents combine scientific excellence, awareness of unmet medical needs, and entrepreneurial spirit.

Christian Chabannon,
MD, PhD
Professor
Aix-Marseille Université
France

Roberto Gramignoli,
MS, PhD
Senior Researcher
Karonlinska Institutet
Sweden
A Mentee’s Perspective
This reinvigorating experience had allowed us to stand ‘upon the shoulders of giants’ to achieve a shining, yet practical vision on contributing to the future of cell and gene therapies.

Christian Chabannon, MD, PhD
Professor
Aix-Marseille Université
France
A Mentor’s Perspective
Diseases know no borders. Global problems need global and new solutions. Developing innovative therapies requires the involvement of committed individuals whose talents combine scientific excellence, awareness of unmet medical needs, and entrepreneurial spirit.
After all, cell and gene therapies are paradigm-shifting. They promise curative efficacy for diseases often unmanageable by existing pharmaceuticals. The ISCT has a core mission in overseeing the translation of proven novel therapies into clinical practice, made possible thanks to its tremendous capacity to gather preeminent experts across all fields of cell and gene therapies worldwide, from academic institutions to regulatory agencies and industry partners. Thanks to visionary educational programs such as the ISCT Mentoring Program, early stage professionals like myself had the terrific opportunity to connect with such experts as mentors to share experience and develop expertise in a collaborative, friendly network.

Roberto Gramignoli,
MS, PhD
Senior Researcher
Karonlinska Institutet
Sweden
A Mentee’s Perspective
This reinvigorating experience had allowed us to stand ‘upon the shoulders of giants’ to achieve a shining, yet practical vision on contributing to the future of cell and gene therapies.
The ISCT Mentoring Program was designed to bring new blood to the fast-growing field of cell and gene therapy. As a mentor, I was lucky enough to connect with two junior investigators who ticked all the boxes; it is an extremely rewarding experience to be part of a global initiative that helps accelerate biomedical progress worldwide.
Our group’s work resulted in a peer-reviewed publication that identifies some major hurdles along the developmental path of new treatments manufactured through cell or tissue engineering.
The ISCT Mentoring Program was designed to bring new blood to the fast-growing field of cell and gene therapy. As a mentor, I was lucky enough to connect with two junior investigators who ticked all the boxes; it is an extremely rewarding experience to be part of a global initiative that helps accelerate biomedical progress worldwide.
Our group’s work resulted in a peer-reviewed publication that identifies some major hurdles along the developmental path of new treatments manufactured through cell or tissue engineering.
After all, cell and gene therapies are paradigm-shifting. They promise curative efficacy for diseases often unmanageable by existing pharmaceuticals. The ISCT has a core mission in overseeing the translation of proven novel therapies into clinical practice, made possible thanks to its tremendous capacity to gather preeminent experts across all fields of cell and gene therapies worldwide, from academic institutions to regulatory agencies and industry partners.
Although a decade has passed since the approval of the first ATMP therapy in Europe, and half of a century since the first successful cell transplant, established ATMP products can be counted on one’s fingers. In our editorial in Cytotherapy, my colleagues and I put the perspective of early stage professionals in dialogue with long-term expert vision, to summarize our discussions throughout the 6 months of the ISCT Mentoring Program towards identifying the main challenges in driving cell and gene therapies to the clinic.
Authored by A. Rotolo, C. Chabannon and R. Gramignoli, this editorial in Cytotherapy 22.2 arose out of productive discussions that started within the 2018-2019 ISCT Mentorship Program, and provides a useful and succinct overview of some of the challenges facing the CGT field today.
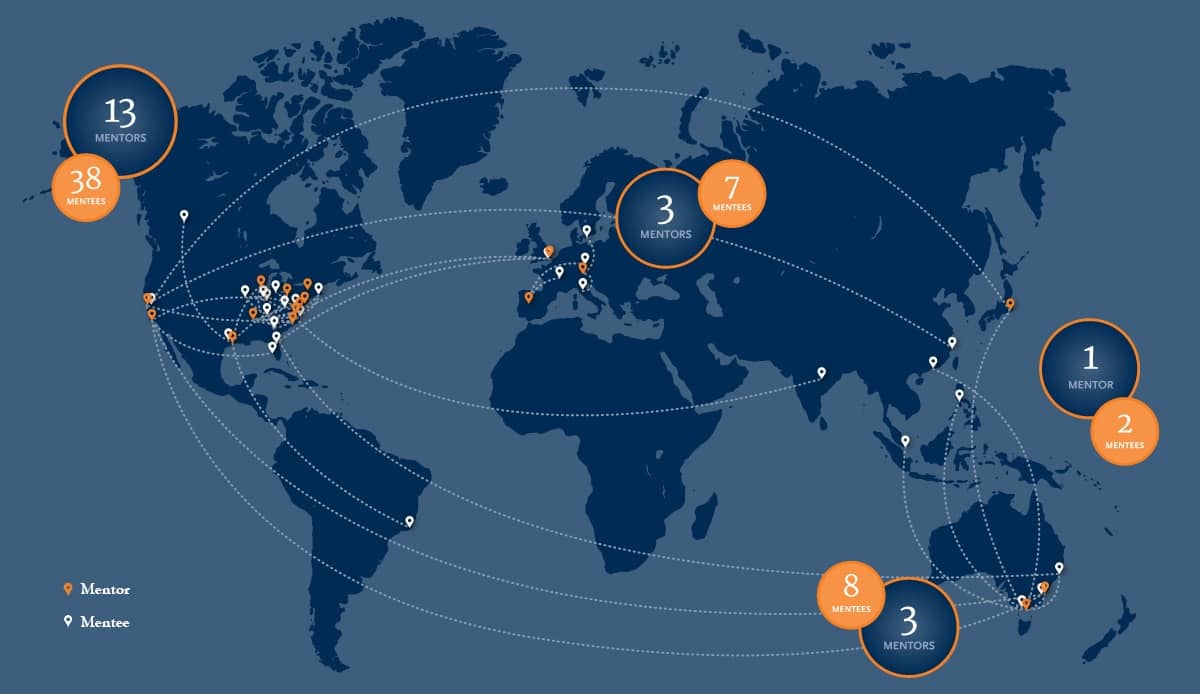
Doubling Down on Mentorship
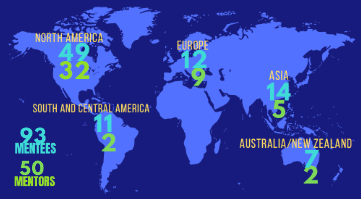
The sophomore year of our ISCT Mentoring program has been met with immense interest across both mentor and mentee groups, almost doubling involvement from our 2018-2019 cycle. With over 20 mentors and 55 mentees for the 2019-2020 roster, our mentorship program is looking to grow even further to promote compassionate and competent expertise across our membership.
Our program is structured to foster a true community of experts, not only serving to connect mentors to mentees, but to create an enduring, collaborative network of mentees who will grow together as they advance both their careers and the field. Each mentor is paired with a small working group of two to three mentees, who work together for a span of at least half a year through monthly teleconferencing. Each mentorship group is determined by areas of interest, including Regulatory, Quality and Operations, Basic Science, Clinical, and Commercialization, as well as by geographical location.
The next cycle of the ISCT Mentoring Program will be recruiting in Q3 2020.
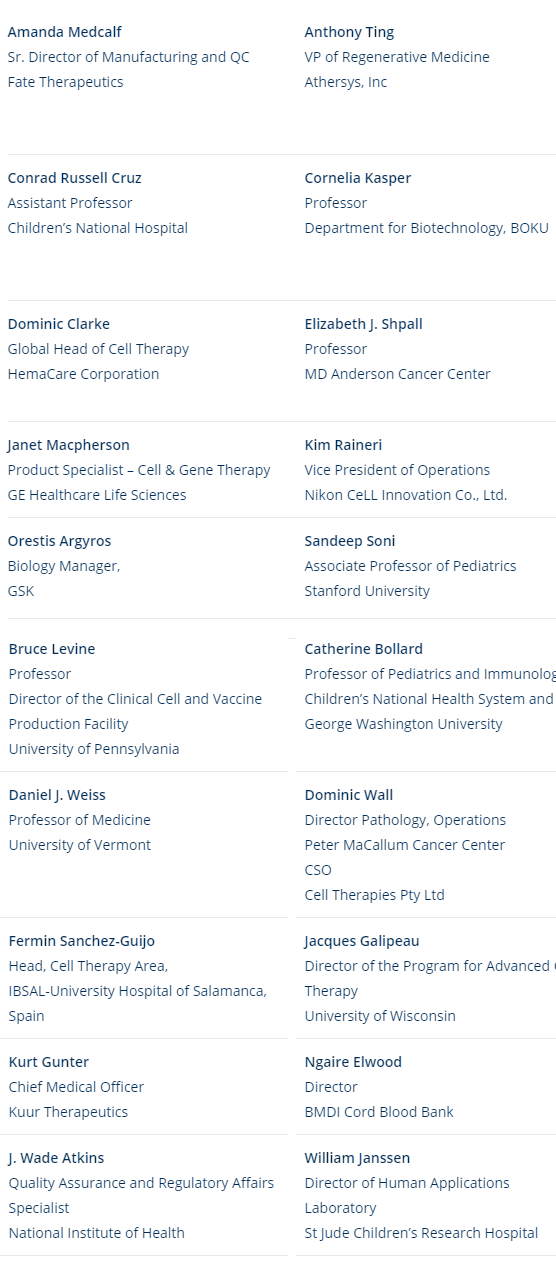
| Amanda Medcalf Sr. Director of Manufacturing and QC Fate Therapeutics | Anthony Ting VP of Regenerative Medicine Athersys, Inc | Bruce Levine Professor Director of the Clinical Cell and Vaccine Production Facility University of Pennsylvania | Catherine Bollard Professor of Pediatrics and Immunology, Children’s National Health System and George Washington University |
| Conrad Russell Cruz Assistant Professor Children’s National Hospital | Cornelia Kasper Professor Department for Biotechnology, BOKU | Daniel J. Weiss Professor of Medicine University of Vermont | Dominic Wall Director Pathology, Operations Peter MaCallum Cancer Center CSO Cell Therapies Pty Ltd |
| Dominic Clarke Global Head of Cell Therapy HemaCare Corporation | Elizabeth J. Shpall Professor MD Anderson Cancer Center | Fermin Sanchez-Guijo Head, Cell Therapy Area, IBSAL-University Hospital of Salamanca, Spain | Jacques Galipeau Director of the Program for Advanced Cell Therapy University of Wisconsin |
| Janet Macpherson Product Specialist – Cell & Gene Therapy GE Healthcare Life Sciences | Kim Raineri Vice President of Operations Nikon CeLL Innovation Co., Ltd. | Kurt Gunter Chief Medical Officer Kuur Pharmaceuticals | Ngaire Elwood Director BMDI Cord Blood Bank |
| Orestis Argyros Biology Manager, GSK | Sandeep Soni Associate Professor of Pediatrics Stanford University | J. Wade Atkins Quality Assurance and Regulatory Affairs Specialist National Institute of Health | William Janssen Director of Human Applications Laboratory St Jude Children’s Research Hospital |

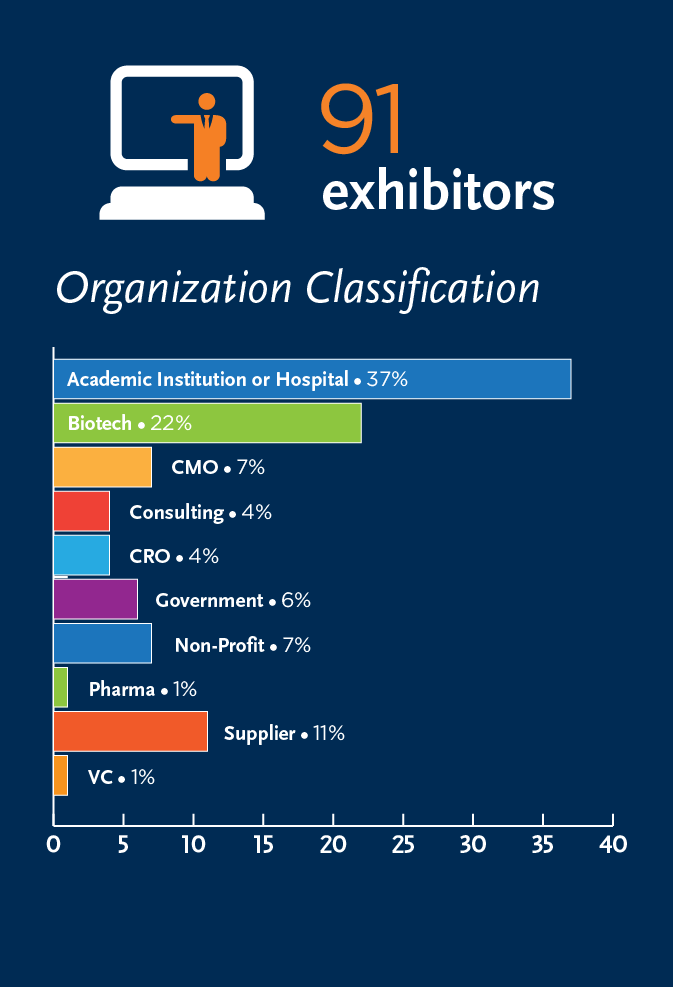
ESP/CellCAN Job Market Survey
During the 2019 ISCT North America Regional Meeting, ISCT’s Early Stage Professionals (ESP) Committee, in partnership with CellCAN, conducted a joint session focused on examining one key bottleneck for the ongoing development of the cell and gene therapy field: A shortage of Highly Qualified Personnel (HQP).
The session made use of live polling to collect data to expands upon the ESP Committee’s 2018 Job Market Survey, sparking an ongoing broader campaign to gather data on the knowledge gaps within the field, as well as the realities of ESPs as they seek career progression.
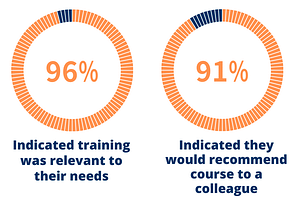
Take a look at some of the results below:
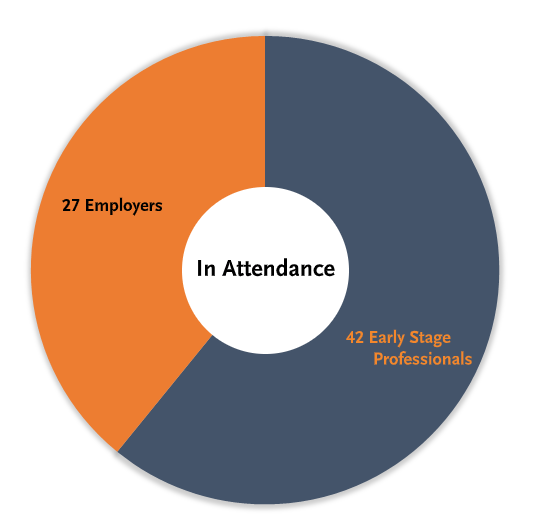
Head-to-Head: Employees vs. Employers
Training Prior to Employment

ESP/CellCAN Job Market Survey
During the 2019 ISCT North America Regional Meeting, ISCT’s Early Stage Professionals (ESP) Committee, in partnership with CellCAN, conducted a joint session focused on examining one key bottleneck for the ongoing development of the cell and gene therapy field: A shortage of Highly Qualified Personnel (HQP).
The session made use of live polling to collect data to expands upon the ESP Committee’s 2018 Job Market Survey, sparking an ongoing broader campaign to gather data on the knowledge gaps within the field, as well as the realities of ESPs as they seek career progression.
Take a look at some of the results below:

Head-to-Head: Employees vs. Employers
Training Prior to Employment

NEW THIS YEAR:

Training the Next Generation
With the dramatic growth in the cell and gene therapy field, there is an urgent need to train the next generation of professionals. ISCT is playing a key role in developing the careers of Early Stage Professionals (ESP) as they enter the CGT field in record numbers. Our ESP membership has grown from 303 in 2018 to 395 in 2019, reflecting a growing interest and engagement within the field.
In 2019, we introduced student registration to our Annual Meeting, as well as to our NA Regional Meeting. This registration category, available to medical, pharmacy, and nursing students hoping to attend ISCT Annual Meetings, includes complimentary pre-conference day registration, in addition to main conference registration at a highly subsidized price of $50. Throughout 2019, 42 students were able to make use of this.
Leading into 2020, ISCT plans to launch a series of exciting training and professional development opportunities. Among these are the European Cell Therapy Training Course, planned for 2021, and a partnered scholarship program for Master’s degree programs offered at the University of Granada and sponsored by Miltenyi Biotec.


John Barrett, MD
Co-Founder, ISCT-ASTCT Cell Therapy Training Course
USA
Cell therapy is an upcoming and unique discipline still forging its trajectory.
It is attracting a broad spectrum of scientists, with both technical and clinical backgrounds who are urgently demanding training in all the aspects of expertise required. This includes not only the basic science behind cellular therapies, but also comprehension of practical steps that have to be taken to make a clinical-grade cell product, as well as knowledge of the regulatory framework covering cell production in both academic and commercial laboratories. “
There is a growing need to train future cell therapists, including experts in cell production as well as those involved in clinical trial development and the translation of cell production from academic institutes to the commercial world. Dave DiGiusto and I conceived of this training course to meet that need. It is a joint effort by the ISCT and the American Society for Cell Therapy (TCT), a connection mirrored in the diversity in backgrounds of attending scholars, many of whom have developed their interest in cell therapy through stem cell transplantation.
In the 5 years since we began, the field has matured, and we have striven to adapt the curriculum to reflect emerging areas of development. We have steadily improved the course, adapting it responsively through feedback from the scholars.
In October 2019 we held the third CTTC in Philadelphia hosted by Dr Bruce Levine. By all accounts, this was our most successful CTTC yet. One shortcoming is that we cannot meet the continually growing demand for training. We can take only a fraction of all the applicants who might benefit from the CTTC and we run the course only on alternating years. We hope that the success of the style and contents of the CTTC will inspire local training courses to develop within ISCT.

John Barrett, MD
Co-Founder, ISCT-ASTCT
Cell Therapy Training Course
USA
Cell therapy is an upcoming and unique discipline still forging its trajectory.
It is attracting a broad spectrum of scientists, with both technical and clinical backgrounds who are urgently demanding training in all the aspects of expertise required. This includes not only the basic science behind cellular therapies, but also comprehension of practical steps that have to be taken to make a clinical-grade cell product, as well as knowledge of the regulatory framework covering cell production in both academic and commercial laboratories.
There is a growing need to train future cell therapists, including experts in cell production as well as those involved in clinical trial development and the translation of cell production from academic institutes to the commercial world. Dave DiGiusto and I conceived of this training course to meet that need. It is a joint effort by the ISCT and the American Society for Cell Therapy (TCT), a connection mirrored in the diversity in backgrounds of attending scholars, many of whom have developed their interest in cell therapy through stem cell transplantation.
In the 5 years since we began, the field has matured, and we have striven to adapt the curriculum to reflect emerging areas of development. We have steadily improved the course, adapting it responsively through feedback from the scholars.
In October 2019 we held the third CTTC in Philadelphia hosted by Dr Bruce Levine. By all accounts, this was our most successful CTTC yet. One shortcoming is that we cannot meet the continually growing demand for training. We can take only a fraction of all the applicants who might benefit from the CTTC and we run the course only on alternating years. We hope that the success of the style and contents of the CTTC will inspire local training courses to develop within ISCT.
A World-Class Standard
Our Cell Therapy Training Course, designed in partnership with the ASTCT and held for the third time in 2019, brought together global leaders and rising stars in the field of cell and gene therapy to advance the field at large. Participating scholars were competitively selected and fully sponsored for the week-long course. Through CTTC, selected scholars were provided with world-class technical, clinical, and leadership training, and connected to an elite network of contacts in the field.
The third biennial 2019 Cell Therapy Training Course was held this fall in Philadelphia, hosted by the University of Pennsylvania. Select lecture recordings from the course can be found here (members only), in addition to the full course program.
If you are interested in enrolling as a scholar, keep an eye out for CTTC 2021.
Cell Therapy Training Course 2019
In Partnership With:
Hosted By:
In Partnership With:

Hosted By:

Sponsored in part by:






CO-CHAIRS

John Barrett, MD

David DiGiusto, PhD
PLANNING FACULTY

Catherine Bollard, MD, MBChB

Colleen Delaney, MD, MSc

Bruce Levine, PhD

Krishna V. Komanduri, MD
SPEAKING FACULTY
Usman Azam, MD
Tmunity Therapeutics
Veronika Bachanova, MD, PhD
University of Minnesota
Joseph A. Fraietta, PhD
Center for Advanced Cellular Therapies
University of Pennsylvania
Perelman School of Medicine
Philip J. Cross, MS
Philip J. Cross & Associates, Inc.
Anna Gilbert, ASQ CQA
BDO USA
Noelle Frey, MD, MSCE
Cell and Transplant Therapy Program
University of Pennsylvania
Elizabeth Hexner MD, MSTR
Abramson Cancer Center
University of Pennsylvania
Whitney Gladney, PhD
Center for Cellular Immunotherapies
University of Pennsylvania
Megan Kasimatis Singleton
JD, MBE, CIP
Office of Human Subjects Research
Johns Hopkins University
Wei-Ting Hwang, PhD
Department of Biostatistics, Epidemiology
and Informatics (DBEI)
University of Pennsylvania
Peter Marks, MD, PhD
Center for Biologics Evaluation and Research (CBER)
U.S. Food & Drug Administration
Lester Lledo, MSN, CRN P
Penn Medicine, Center for Cellular Immunotherapies
University of Pennsylvania
Jos Melenhorst, PhD
Pathology & Laboratory Medicine
University of Pennsylvania
Shannon Maude, MD, PhD
Medical Director, Center for Cellular Immunotherapies
University of Pennsylvania
Donald M. O’Rourke, MD
The Abramson Cancer Center and
Perelman School of Medicine
Doug Olson, PhD
BUHLMANN Diagnostics Corp.
Cancer survivor and patient two in the initial CART 19 clinical trial
Johannes van der Loo, PhD
Center for Cellular & Molecular Therapeutics
The Children’s Hospital of Philadelphia
Elizabeth J. Shpall, MD
The University of Texas MD Anderson Cancer Center
Gabriela Plesa, MD, PhD
Center for Cellular Immunotherapies
University of Pennsylvania
SCHOLARS

Gabor Foldes
MD, PhD

Giulia Golinelli
PhD

Andrea Henden
MBBS(Hons), FRACP, FRCPA

Gaurav Sutrave
BSc(Med)/MBBS(Hons I), FRACP, FRCPA

Robert Myles Wright
MBBS

Saurabh Dahiya
MBBS

Shoba A. Navai
MD

Shabnum Patel
PhD

Irene Scarfo
PhD

Frederico Simonette
MD, PhD

Mauro Castellarin
PhD

Saba Ghassemi
PhD

Philipp C Rommell
Dr.rer.nat
The Grassroots Footprint of our Regional Model
In 2019, we revised our regional engagement practices across the globe, both to widen our footprint in the world and to engage specific needs in specific places within the field of Cell and Gene Therapy.
Our regional model is unique within the field of cell and gene therapy. Our membership benefits from the passionate involvement of local leadership across the globe, with independent initiative that is supported by our head office. Our committees conduct region-focused research, create partnerships with other organizations on a global scale, and host scientific meetings and sessions that continually push the field forward.
This is accomplished through the hard work of our five autonomous regional committees, and the support of ISCT’s global network. Throughout the year, the efforts of each committee have resulted in a series of productive scientific meetings, partnered joint sessions, and growth both in our membership and in our connection to the field.
Events created by our regional committees are vital towards our efforts to coordinate scientific advancement, regulatory oversight, and innovative commercialization strategies at a global level.
Here are some of the highlights from our regional committee events this year:

Steve Oh, PhD
Past Regional Co Vice-President, ISCT Asia
Singapore
Excitement and Challenges in Asia
The growth of immunotherapies has resulted in excitement and challenges in Asia. As these breakthrough therapies become widespread, challenges like the cost of goods, manufacturing, and critical quality attribute (CQA) issues will have to be addressed.
At the SCSS-ISCT Joint Meeting, “Frontiers in Cell Therapy,” presentations from ISCT experts like Ohad Karnelli, and Bruce Levine certainly drew delegate attention. Company presentations from partners like RoosterBio, BioSpherix, and X-Therma also provided practical and timely solutions for the field.
Three programmes from Singapore, funded at a total of S$80 million, were also highlighted at the event. In 2020, we expect to see some industry engagements being announced as a result of this significant investment by the Singapore government.
Showcasing Science Down Under
The ISCT ANZ-ASSCR-AGCTS Joint Scientific Meeting provided me with the wonderful opportunity to present our group’s work in targeted T cell immunotherapies to an engaged and dynamic group of peers. The conference size and format facilitated discussion between participants across disciplines. The scientific connections and discourse generated by presenting at this focused meeting will be invaluable to my professional development and future work.
I was honoured to be the recipient of an ISCT ANZ travel grant and award for highest scoring abstract. Regional opportunities such as the this grant are so important to early career researchers to facilitate the sharing of innovative work and continue to grow our community of expert, passionate scientific minds.

Wei Jiang, PhD
Westmead Cellular Therapies Group
Australia
Our Regional Leaders
Oscar Lee, MD, PhD
Regional Vice-President
ISCT Asia
Click here to visit our site
Ngaire Elwood, PhD
Regional Vice-President
ISCT ANZ
Click here to visit our site
Joan Garcia-Lopez, MD, PhD
Regional Vice-President
ISCT Europe
Click here to visit our site
Lynn O’Donnell, PhD
Regional Vice-President
ISCT North America
Click here to visit our site
Patricia R.M. Rocco, MD, PhD
Regional Vice-President
ISCT SCA
Click here to visit our site
Oscar Lee, MD, PhD
Regional Vice-President
ISCT Asia
Click here to visit our site
Ngaire Elwood, PhD
Regional Vice-President
ISCT ANZ
Click here to visit our site
Joan Garcia-Lopez, MD, PhD
Regional Vice-President
ISCT Europe
Click here to visit our site
Lynn O’Donnell, PhD
Regional Vice-President
ISCT North America
Click here to visit our site
Patricia R.M. Rocco, MD, PhD
Regional Vice-President
ISCT SCA
Click here to visit our site
Oscar Lee, MD, PhD Regional Vice-President ISCT Asia
Click here to visit our site
Ngaire Elwood, PhD Regional Vice-President ISCT ANZ
Click here to visit our site
Joan Garcia-Lopez, MD, PhD Regional Vice-President ISCT Europe
Click here to visit our site
Lynn O’Donnell, PhD Regional Vice-President ISCT North America
Click here to visit our site
Patricia R.M. Rocco, MD, PhD Regional Vice-President ISCT SCA
Click here to visit our site
Oscar Lee, MD, PhD
Regional Vice-President
ISCT Asia
Click here to visit our site
Ngaire Elwood, PhD
Regional Vice-President
ISCT ANZ
Click here to visit our site
Joan Garcia-Lopez, MD, PhD
Regional Vice-President
ISCT Europe
Click here to visit our site
Lynn O’Donnell, PhD
Regional Vice-President
ISCT North America
Click here to visit our site
Patricia R.M. Rocco, MD, PhD
Regional Vice-President
ISCT SCA
Click here to visit our site
Cytotherapy, Rebranded

Donald Phinney, PhD
Senior Editor, Cytotherapy
United States
Cutting edge research in the rapidly expanding fields of cell and gene therapy
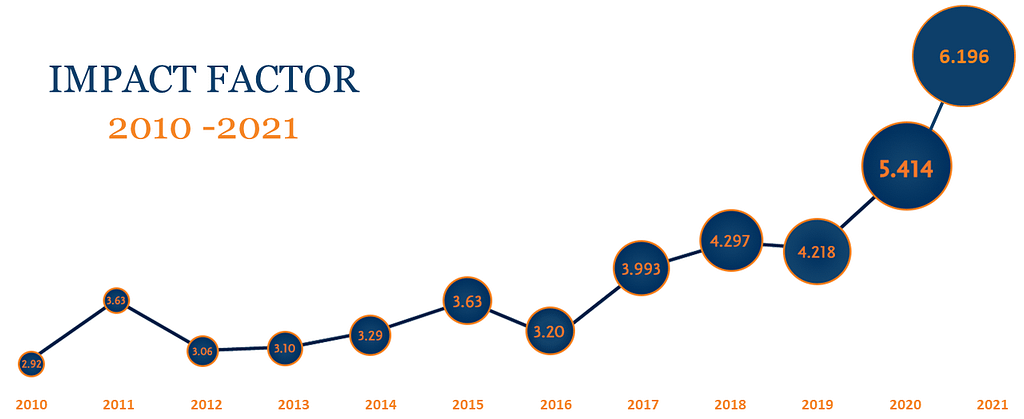
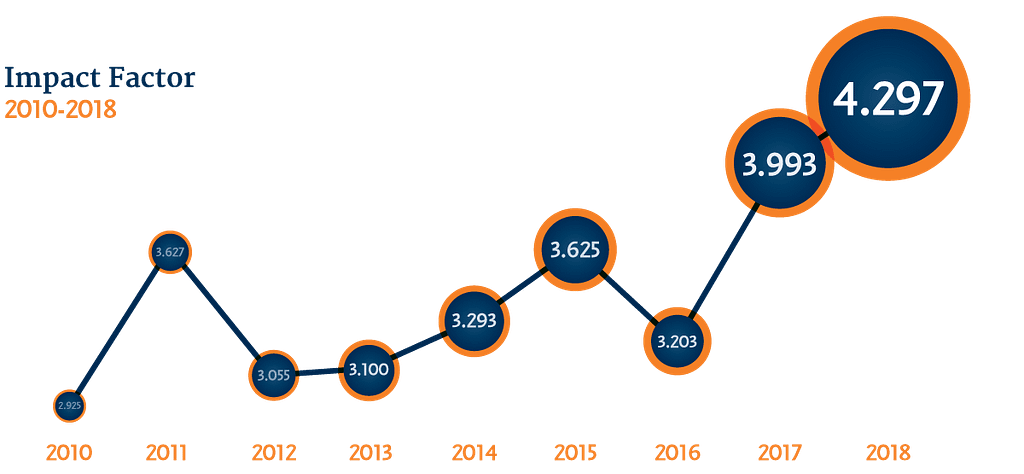
Cytotherapy has undergone remarkable changes in aesthetic and content and celebrated a record impact factor in 2019. Building on this momentum, the Editorial Team is focused on continuing to increase the global influence of the journal by publishing cutting edge research in the rapidly expanding fields of cell and gene therapy. They are also working to further strengthen the relationship between the journal and ISCT by developing new initiatives, collaborations, and special features that highlight the important work done by its members. Through these efforts, we hope to see unprecedented rates of growth for both the journal and the society over the next few years.
New Look. New Editorial Board. New Impact.
2019 has been an exciting year for Cytotherapy. In June, our publication was rebranded on several levels. First, the look and feel of the journal has been updated to reflect its expanding focus. Photographs of cells, sourced from our society’s membership through the Insta-Your-Cells photo challenge, speak to a continuing commitment to engage with our community.
The expertise of Cytotherapy has also shifted, with new additions to our editorial staff, and the clarification of the journal’s scope and submission guidelines. Cytotherapy, in line with the society’s vision towards more cohesive and committed communications, has developed upon its respectable foundations.
In 2019, Cytotherapy‘s record impact factor of 4.297 was also reported for 2018. This number, derived from ScienceDirect, Elsevier’s online portal, measures “the average number of citations received in a particular year by papers published in the journal during the two preceding years” (Clarivate Analytics, 2019).

The 2019 Cytotherapy Editorial Board
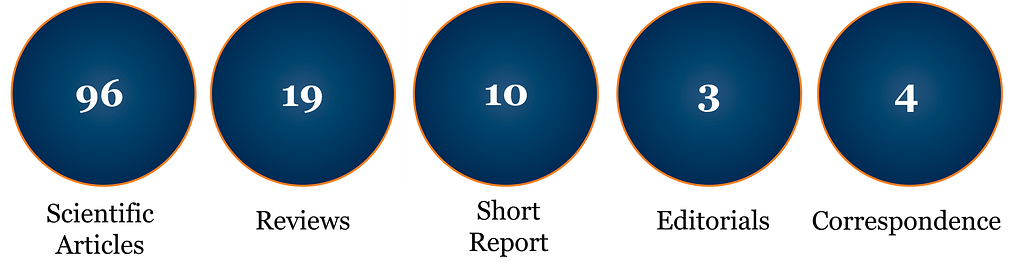
Cytotherapy, the official journal of the ISCT, publishes novel and innovative results from high quality scientific and clinical studies in the fields of cell and gene therapy. Studies evaluating the potency of experimental cell and gene therapies in clinically relevant animal models of disease and describing important advances in cell/gene-based product manufacturing and validation are welcomed. Results of clinical studies evaluating the safety and efficacy of cell and gene therapies in early and late phase trails are also of interest. In addition to short reports and full-length articles, the journal also accepts editorials addressing emerging trends and potential controversies in the field, and review articles summarizing bodies of work that have made lasting impacts in the field.
Senior Editor

Donald G. Phinney, PhD
Scripps Florida
Jupiter, Florida
United States
Associate Editors
Massimo Dominici, MD
University of Modena and Reggio Emilia, Modena, Italy
Oscar Lee, MD, PhD
National Yang-Ming University, Taipei, Taiwan
Luis Ortiz, MD
University of Pittsburgh, Pittsburgh, Pennsylvania, United States
John Rasko, MBBS, PhD
Royal Prince Alfred Medical Centre, Newtown, Australia
Sowmya Viswanathan, PhD
University Health Network, Toronto, Ontario, Canada
Editorial Board Members
2019 marked the start of the ISCT Talking With Giants series.
Featured at the start of several issues of Cytotherapy, this inspirational series provides both a celebration of the contributions of scientific leaders in cell and gene therapy, and an opportunity for readers to learn more about the passion, motivations, and visions of these giants within an informal question and answer format.
ISCT Talking With Giants 2019
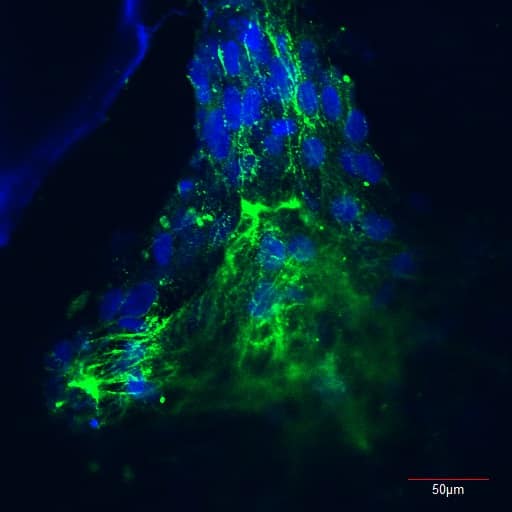
Inaugural Insta-Your-Cell Photo Challenge
1st Place
Imam Rosadi (Indonesia)
2nd Place
Ahmad Galuta (Canada)
3rd Place
Saba Ghassemi (United States)
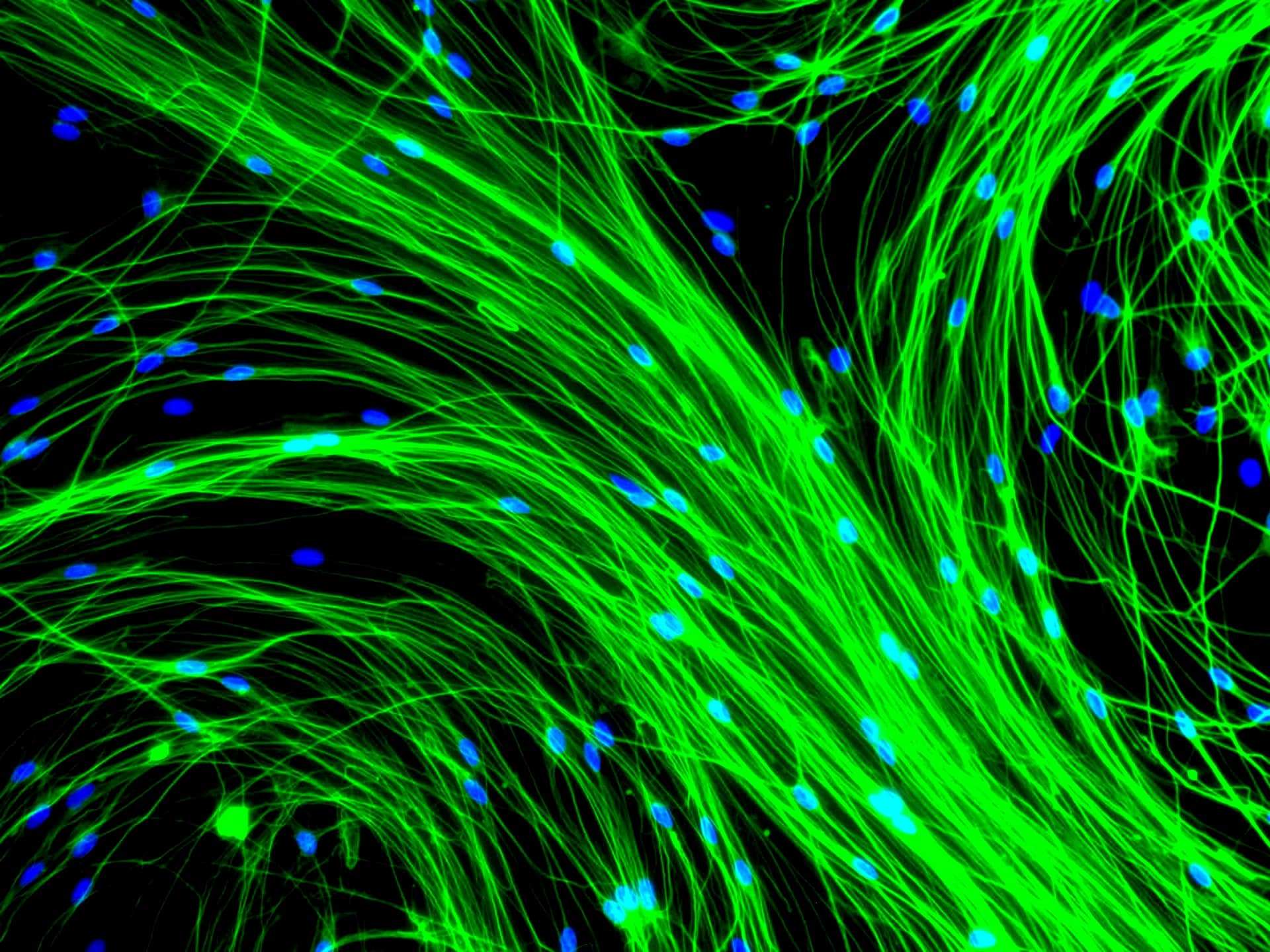
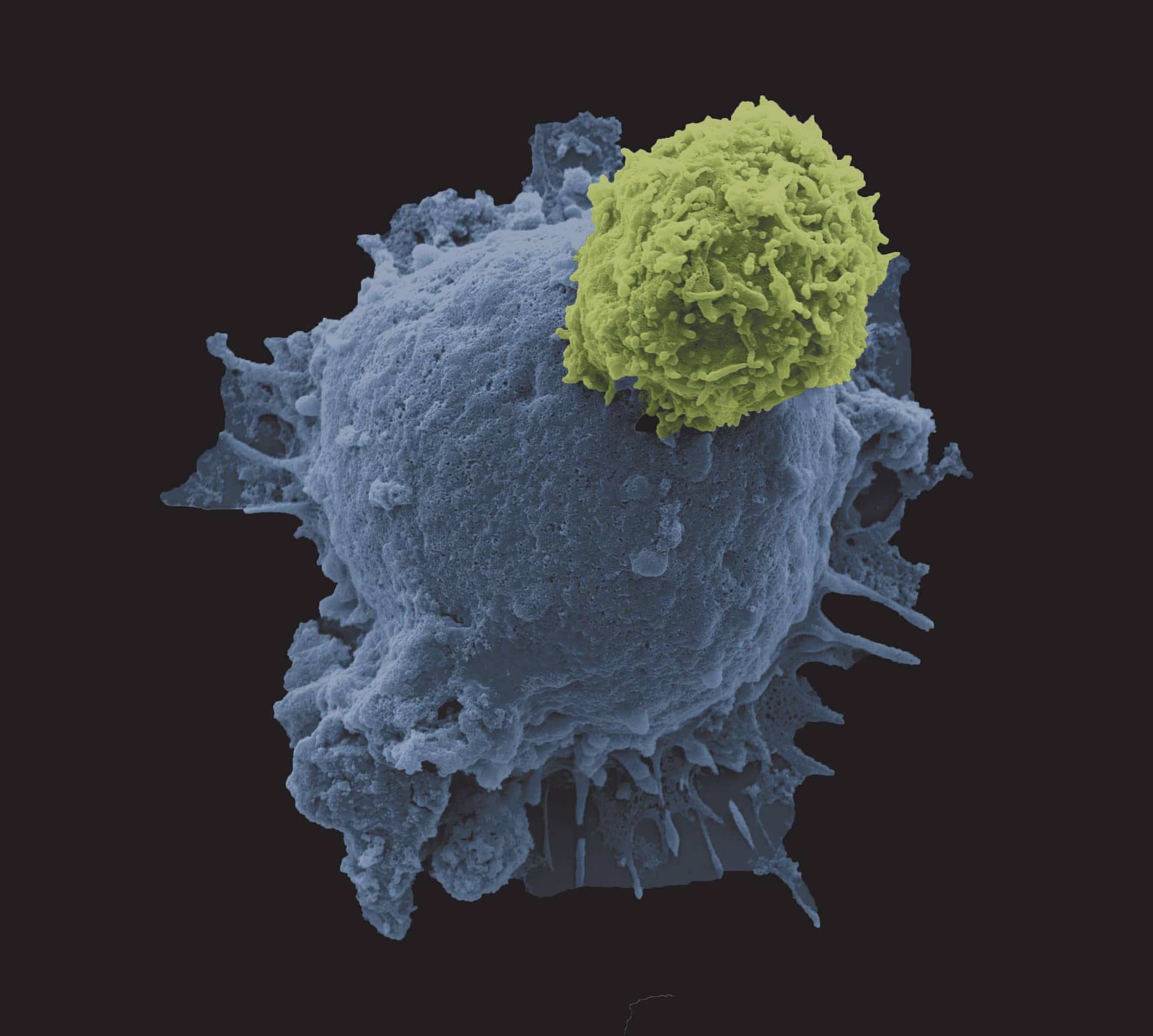
The Insta-Your-Cells photography competition showcased images taken in laboratory environments by our members. This competition featured over 50 unique and fascinating photos taken at the cellular level, and served to visualize some aspects of the intricate work that make scientific advancement possible in our field.
Winners are featured on the front covers of Cytotherapy from June 2019 onwards, with the 1st place winner being featured in January 2019.
Additional prizes included complimentary conference registration (1st place), and pre-conference day registration (2nd and 3rd place)Our Three Pillars




ISCT’s Committees represent an invaluable network of dedicated scientific leaders that work to develop and deliver papers that advance the field of cell and gene therapy. Below are a featured series of their publications throughout this year.
For more information on contributing to one of our committees, take a look at our Committee Directory.
Co-Chaired By:
| Rachele Ciccocioppo, MD University of Verona, Verona, Italy | Giuseppe Orlando, MD, PhD Wake Forest University, Winston-Salem, NC, USA |
Perspectives of the International Society of Cell & Gene Therapy Gastrointestinal Scientific Committee on the Intravenous Use of Mesenchymal Stromal Cells in Inflammatory Bowel Disease.
Authors: Ciccocioppo R, Baumgart DC, Dos Santos CC, Galipeau J, Klersy C, Orlando G.
Chaired By:
Jacques Galipeau, MD |
Mesenchymal stem versus stromal cells: International Society for Cellular Therapy Mesenchymal Stromal Cell committee position statement on nomenclature.
Authors: Viswanathan Y, Shi Y, Galipeau J, Krampera M, Leblanc K, Martin I, Nolta J, Phinney DG, Sensebe L.
Manufacturing mesenchymal stromal cells for clinical applications: A survey of Good Manufacturing Practices at U.S. academic centers.
Authors: Phinney DG, Galipeau J.
Challenges for mesenchymal stromal cell therapies.
Authors: Martin I, Galipeau J, Kessler C, Leblanc K, Dazzi F.
Co-Chaired By:
Sai-Kiang Lim, PhD | Bernd Giebel, PhD |
Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications
Authors: Witer K, Van Balkom, B, Bruno S, Choo A, Dominici M, Gimona M, Hill A, De Klejin D, Koh M, Lai R, Mitsialis S, Ortiz L, Rohde E, Asada T, Toh W, Weiss D, Zheng L, Giebel B, Lim S.
Co-Chaired By:
(2018-2020) Kenneth Micklethwaite, MD The Westmead Institute for Medical Research Sydney, Australia | (Jan 2018 – Present) Patrick Hanley, PhD Children’s National Medical Center Washington, DC, United States |
Advancing cellular therapies towards standard of care: a focus on testing of cellular therapy products
The field of cell and gene therapy has rapidly grown from a number of small, isolated centers 20 years ago to specialized cell and gene therapy centers worldwide. Nonetheless, despite the recent high-profile acquisitions of small companies developing cell therapies such as chimeric antigen receptor (CAR)-T products, the bulk of developments in this field remain led by academic drug developers, most of whom are focused on a treatment for their group of patients with local production and supply and are not aware of the challenges of supplying the national or global patient need.
Critical testing and parameters for consideration when manufacturing and evaluating tumor–associated antigen-specific T cells
Authors: Tanna J, Ulrey R, Williams K, Hanley P.
The past year has seen remarkable translation of cellular and gene therapies, with U.S. Food and Drug Administration (FDA) approval of three chimeric antigen receptor (CAR) T-cell products, multiple gene therapy products, and the initiation of countless other pivotal clinical trials. What makes these new drugs most remarkable is their path to commercialization: they have unique requirements compared with traditional pharmaceutical drugs and require different potency assays, critical quality attributes and parameters, pharmacological and toxicological data, and in vivo efficacy testing. What’s more, each biologic requires its own unique set of tests and parameters…
Mesenchymal stromal cell therapy: progress in manufacturing and assessments of potency
Authors: Robb K, Fitzgerald J, Barry F, Viswanathan S.
Mesenchymal stromal cell (MSC) therapies have been pursued for a broad spectrum of indications but mixed reports on clinical efficacy have given rise to some degree of skepticism regarding the effectiveness of this approach. However, recent reports of successful clinical outcomes and regulatory approvals for graft-versus-host disease, Crohn’s disease and critical limb ischemia have prompted a shift in this perspective. With hundreds of clinical trials involving MSCs currently underway and an increasing demand for large-scale manufacturing protocols, there is a critical need to develop standards that can be applied to processing methods and to establish consensus assays for both MSC processing control and MSC product release…
Tumor-infiltrating lymphocytes: Streamlining a complex manufacturing process
Authors: Hopewell E, Cox C, Pilon-Thomas S, Kelley L.
Characterization of human natural killer cells for therapeutic use
Authors: Wagner A, Alici E, Lowdell M.
As a part of the innate immune system, natural killer (NK) cells are cytotoxic lymphocytes that can exert cytotoxic activity against infected or transformed cells. Furthermore, due to their expression of a functional Fc receptor, they have also been eluded as a major effector fraction in antibody-dependent cellular cytotoxicity. These characteristics have led to multiple efforts to use them for adoptive immunotherapy against various malignancies. There are now at least 70 clinical trials testing the safety and efficacy of NK cell products around the world in early-phase clinical trials…
Manufacturing chimeric antigen receptor T cells: issues and challenges
Authors: Roddie C, O’Reilly M, Dias Alves Pinto J, Vispute K, Lowdell M.
Clinical trials of adoptively transferred CD19 chimeric antigen receptor (CAR) T cells have delivered unprecedented responses in patients with relapsed refractory B-cell malignancy. These results have prompted Food and Drug Administration (FDA) approval of two CAR T-cell products in this high-risk patient population. The widening range of indications for CAR T-cell therapy and increasing patient numbers present a significant logistical challenge to manufacturers aiming for reproducible delivery systems for high-quality clinical CAR T-cell products. This review discusses current and novel CAR T-cell processing methodologies and the quality control systems needed to meet the increasing clinical demand for these exciting new therapies.
T-cell receptor gene-modified cells: past promises, present methodologies and future challenges
Authors: Rego R, Morris E, Lowdell M.
Immunotherapy constitutes an exciting and rapidly evolving field, and the demonstration that genetically modified T-cell receptors (TCRs) can be used to produce T-lymphocyte populations of desired specificity offers new opportunities for antigen-specific T-cell therapy.
Overall, TCR-modified T cells have the ability to target a wide variety of self and non–self targets through the normal biology of a T cell. Although major histocompatibility complex (MHC)–restricted and dependent on co-receptors, genetically engineered TCRs still present a number of characteristics that ensure they are an important alternative strategy to chimeric antigen receptors (CARs), and high-affinity TCRs can now be successfully engineered with the potential to enhance therapeutic efficacy while minimizing adverse events…
Chemistry, manufacturing and controls for gene modified hematopoietic stem cells
Authors: Soni S, Kohn D.
Gene modification of hematopoietic stem cells is increasingly becoming popular as a therapeutic approach, given the recent approvals and the number of new applications for clinical trials targeting monogenetic and immunodeficiency disorders. Technological advances in stem cell selection, culture, transduction and gene editing now allow for efficient ex vivo genetic manipulation of stem cells. Gene-addition techniques using viral vectors (mainly retrovirus- and lentivirus-based) and gene editing using various targeted nuclease platforms (e.g., Zinc finger, TALEN and Crispr/Cas9) are being applied to the treatment of multiple genetic and immunodeficiency disorders. Herein, the current state of the art in manufacturing and critical assays that are required for ex vivo manipulation of stem cells are addressed. Important quality control and safety assays that need to be planned early in the process development phase of these products for regulatory approval are also highlighted.
Proposal for the International Society for Cell & Gene Therapy position statement on assays for the quality control and potency assessment of adoptive cellular immunotherapies
Authors: Weil B, Hanley P, Lowdell M.
Translation of cell and gene therapies from pre-clinical experiments to clinical trials and final drug licensing brings requires the development, verification and even validation of the assays essential for the definition of the drug product. The technical and scientific challenges in doing this are far greater than they seem at first and are compounded by a lack of approved standards for assays used to support (c)GMP manufacture. This paper highlights some of those challenges and proposes solutions based on the experience of our colleagues using similar assay platforms in regulated pathology laboratories.
Organized by the ISCT Immuno and Gene Therapy Committee
Chaired by Sandeep Soni, MD, Stanford University, Palo Alto, CA, United States
The ISCT Scientific Signature Series offers the unique opportunity to gather key opinion leaders in the chosen field to discuss, present and develop position statements to move the field forward. These events are aimed at driving thought leadership by providing content and building collaboration around concept papers, consensus statements, clinical networks, regulatory proposals, and recommendations for investment in clinical and basic research in cell and gene therapy medicine.
ISCT’s North America Regional Committee 2019 Meeting in Madison, USA featured our annual scientific signature series – a full-day symposium event that gathered key opinion leaders in Gene Therapy to drive the field forward through the development and discussion of position statements in a public forum.
This year’s Scientific Signature, “Novel Ex-Vivo Gene Therapy Approaches and Emerging Gene Editing Technologies” focused on perspectives from translational science, manufacturing, and regulation. The symposium consisted of four sessions, each consisting of three topic presentations, followed by a thirty minute open discussion:
- HSPC Selection, Conditioning, and the Synthetic Biology Platform
- The CRISPR-CAS Gene Editing System
- Advances in Gene Therapy Technologies and Applications
- Regulatory & Manufacturing Considerations
Sponsors of ISCT’s Scientific Signature Series 2019






The International Society for Cell and Gene Therapy (ISCT) and the AABB have an ongoing series of projects overseen by the ISCT-AABB Joint Working Group. The main project team managed by the Joint Working Group focuses on research on pooled Human Platelet Lysate (pHPL).
In 2019, the project team released an inaugural jointly-published manuscript across both societies’ peer-reviewed journals: Cytotherapy and Transfusion.
Alongside this exciting progression, 2019 marks a year of expansion for the working group, as a second project team, focusing on biopreservation, has been established to advance cellular therapy product stability. For more information, read Lizette Caballero’s article in the February 2019 issue of Telegraft and the AABB’s update document.
ISCT’s scientific community operates on a global scale, providing expert review and support in the interest of proven and safe research practices.
Through liaison work with standards organizations, a broader scientific community, and with investigatory stakeholders, ISCT conducts thought leadership in the field of cell and gene therapy on a global scale. Below is a snapshot of some actions taken by ISCT committees over the past year:
TOTAL
INTERNATIONAL
International Standards Organization
MSC and Stromal Cell Definitions
International Standards Organization*
Standards for UC-MSC Banking
Standards for BM-MSC Banking
*ISCT has provided ongoing liaison work for two biobanking standards documents, providing consultation around nomenclature for the international standards organization
NORTH AMERICA
Re: Fetal Tissue Research Amendment
Letter of Support to Mark Pecan of the HHS
Re: NIH Requirements for Fetal Tissue Research
ISSCR-led Scientific Coalition Letter
Re: NIH Requirements for Fetal Tissue Research
US Senate Member-led Letter
ISCT offers regulatory review and consultation from cell and gene therapy experts for regulatory authorities worldwide.
Review and recommendations are made by our global task force, as well as by regional Legal and Regulatory Affairs Committees in our North America, Europe, and Australia and New Zealand regions. Each consultation consists of significant comments, suggested revisions, and edits for regulatory documentation. Below are a series of major guidance reviews from 2019:
TOTAL
INTERNATIONAL
Stakeholder Consultation for PIC/S GMP Guide
Annex 2A – MANUFACTURE OF ADVANCED THERAPY MEDICINAL 3 PRODUCTS FOR HUMAN USE
Annex 2B – MANUFACTURE OF BIOLOGICAL MEDICINAL 4 SUBSTANCES AND PRODUCTS FOR HUMAN USE
NORTH AMERICA
ISCT-Endorsed Letter
Letter of Recommendations to the HRSA regarding the Zika Virus
EUROPE
European Medicines Agency
Guideline on quality, non-clinical and clinical requirements for investigational ATMP in CT (EMA-CAT-852602-2018)
European Medicines Agency
Guideline on quality, non-clinical and clinical aspects of medicinal products containing genetically modified cells (EMA/CAT/GTWP/671/2008)
AUSTRALIA AND
NEW ZEALAND
NEW ZEALAND
Australian National Gene Technology Scheme
Implementing Recommendations of the Third Review of the Gene Technology Scheme: Phase 1 Consultation
ISCT hosts liaison meetings for stakeholders in the field of cell and gene therapy to coordinate with national regulatory authorities. Each of these liaison meetings consists of a series of focused presentations on the recent developments and urgent concerns that concern stakeholders in the community and industry of cell therapy. These presentations are directed both at regulatory authorities and at fellow stakeholders in attendance, and are designed to promote up-to-date and comprehensive policy development.
Throughout 2019, two series of liaison meetings held their latest iteration:
Since 2004, ISCT has led over twenty invited stakeholder organizations in an annual face-to-face meeting with the FDA Cell Therapy Liaison Meeting (CTLM).
This year’s presentations consisted of:
Regulation of Biobanking Material for Future Products
Mahendra Rao, MD, PhD
Collection of MNCs for Immune Effector Cells
Jay Raval, MD
Minimum Characterization Criteria for Clinical Grade iPSC Products
Aisha Khan, MS, MBA
Early Interaction Mechanisms for Tool/Device Developers
Michael Mendicino, PhD
Since 2014, the Health Canada Biologics and Genetic Therapies Directorate has hosted regular bilateral meetings with the Cell Therapy Stakeholder Group to promote regulatory dialogue that identifies critical information in easing the translation of cell therapy and regenerative medicine innovations into Canadian healthcare.
Co-Chaired By:
Karen Nichols, Esq.VP, Regulatory and Quality, Magenta Therapeutics, United States | Dominic Wall, PhD, FFSc(RCPA)CSO Cell Therapies Pty Ltd, Peter MacCallum Cancer Centre, Australia |
The ISCT Global Regulatory Perspectives (GRP) workshop represents the diverse activities and partnerships between international regulatory bodies, industry, clinicians and academia. Steered by representatives from the Society’s Legal and Regulatory Affairs Committees from Asia, Australia, Europe, North America, and South and Central America, the GRP workshop takes place once every year as a featured full-day event during the ISCT’s Annual Scientific Meeting. With regulators in attendance from every region of ISCT membership, GRP programming targets a truly global perspective while maintaining focus on key emergent issues in the cell and gene therapy field.
Each GRP Workshop consists of alternating speaker sessions and case study-based panel discussions.
During ISCT 2019 Melbourne, the GRP Workshop covered topics including the following:
- CMC Considerations (including testing) for Pediatric Treatments
- Risk Assessment for Administration of Non-conforming Products
- Clinical Strategies for Treatment of Pediatric Diseases
- Regulatory Convergence

Catered to the industry community within the field of cell and gene therapy, the ISCT Commercialization Signature Series provides an overview and focused discussion of the state of market access and adoption for the sector. Each signature event is designed to bring not only leading positions and information into discussion, but also to facilitate networks across key peers in academic and industry positions.
The third ISCT Commercialization Committee Signature Series was held this past January at the Phacilitate Cell & Gene Therapy World 2019 event in Miami. Titled “Patients First: Achieving Global CGT Adoption,” the session focused on strategies and positions tailored to leveraging the increasingly robust investments, interest, and scientific advancement in CGT to successful manufacturing, distribution, and legislative approval.
This timely conference session focused on three general areas:
1. The business of cell & gene therapy: state of the financial markets
2. Getting the product to the patient: global manufacturing strategies
3. Overcoming cell & gene therapy adoption barriers: positioning clinical evidence and pricing models for success
Sponsored by our 2019 Industry Members



Membership: 49% outside of the United States from 60+ countries
Regional Forum and Meeting Partners
ISCT ANZ-ASSCR-AGCTS
Joint Scientific Meeting
ISCT SCA Regional Forum at SOMICET Annual Congress
SCSS-ISCT
Joint Symposium
ISCT ANZ-ASSCR-AGCTS
Joint Scientific Meeting

ISCT SCA Regional Forum at SOMICET Annual Congress


SCSS-ISCT
Joint Symposium

Co-Founded Organizations


Strategic Liaisons




















Webinar Sponsors
October 30
November 21
March 27





October 30


November 21

March 27


Joint Session Partners










Regional Forum and Meeting Partners
ISCT ANZ-ASSCR-AGCTS
Joint Scientific Meeting
ISCT SCA Regional Forum at SOMICET Annual Congress
SCSS-ISCT
Joint Symposium
ISCT ANZ-ASSCR-AGCTS
Joint Scientific Meeting

ISCT SCA Regional Forum at SOMICET Annual Congress


SCSS-ISCT
Joint Symposium

Co-Founded Organizations


Strategic Liaisons



















Webinar Sponsors
October 30
November 21
March 27





October 30


November 21

March 27


Joint Session Partners










A Legacy of Engagement
Since 1992, ISCT has had connected more than 17,000 delegates through our Annual Meetings and communicated with more than 30,000 cell and gene therapy professionals at cutting-edge meetings, events, webinars and seminars to translate the advancement of research into clinical adoption and standard of care over the past 26 years.

ISCT 2019 Melbourne
Chaired By:
Ngaire Elwood
Kunihiko Suzuki
Siok Tey
Ngaire | Kunihiko | Siok |
 |  |  |
Every year, the ISCT Annual Meeting delivers a highly curated scientific program showcasing the latest clinical results and technologies expanding global access to new and emerging cell and gene therapies.

TELEGRAFT LIVE
Making Connections Before the Meeting
The Meeting is Underway!
Scientific, Industry, and ESP Networking
The Celebrations Continue
“THE CELLFIE”

ISCT Regional Meetings 2019
ISCT NA Regional Meeting
September 13-14 – Madison, WI, USA
“Engineering the Future of Cell and Gene Therapies”
ASSCR-ACGTS-ISCT Joint Scientific Meeting:
November 13 – Brisbane
“From Stem Cells to Genes to Therapies”
ISCT SCA At 5th SOMICET Congress
October 21-23 – Mexico City
“5th International Meeting on Stem Cells and Regenerative Medicine”
March 27, 2019
A Journey to Next Generation Manufacturing Solutions
Total registered: 238

Organized by the ISCT Process and Product Development Subcommittee
April 25, 2019
and Decision-Making Criteria for CGT Investments
Total registered: 82

Organized by the ISCT Business Models & Investment Subcommittee
July 17, 2019
October 23, 2019
Dr. Joanne Kurtzberg
Total registered: 41

Organized by the
ISCT Early Stage Professional (ESP) Committee
October 30, 2019
Cell & Gene Therapies: Addressing the Impact of Human Serum and Serum Alternatives
Total registered: 144

Organized by the ISCT Process and Product Development Subcommittee
November 21, 2019
Best Practices for Designing Cell & Gene Therapy Clinical Trials
Total registered: 200

Organized by the ISCT Market Access & Patient Advocacy Subcommittee
To address patient uncertainty around cell and gene therapy treatments throughout the explosive growth of the field, ISCT’s Presidential Task Force (PTF) on the Use of Unproven and/or Unethical Cell and Gene Therapies works to provide reliable and verified information to a global public.
Since its inception, the PTF has consolidated several up-to-date resources for the public regarding the field of cell and gene therapy:
Throughout this past year, the PTF took action on several issues around unproven cell therapy treatments and procedures. Chief among these was the issue of cell banking, on which the PTF issued a Statement of Concern on October 21st. Following this, the PTF took action on November 13th, creating a consortium of professional and educational societies to provide oversight and to protect the development of legitimate cell therapy products so as to preserve the public’s trust in this growing field.
Finally, on March 27th, members of the ISCT PTF co-authored a publication critically discussing the current status of cell therapies for lung disease, “Translating Basic Research into Safe and Effective Cell-based Treatments for Respiratory Diseases “
Patient Reference Document
This global guide addresses the issue of unproven cell and gene therapies, which are currently being advertised, sold and administered to patients, although they fail to achieve recognized medical standards of proof for safety or efficacy. These unproven treatments are often expensive and offered outside the coverage of routine clinical care. They are not part of a conventional clinical trial and lack regulatory oversight. With this document, the ISCT Presidential Task Force hopes to educate patients and health care professionals, providing resources to determine whether a therapy has been deemed safe and effective by the appropriate governing bodies.
CGT Market Authorization – 2019 List
The ISCT CGT Market Authorization Report is a regularly updated document that will be republished yearly and available online.
This report is the result of an increase in the number of CGT market authorizations, as well as an increase in unproven approaches where cells are delivered as treatments without rigorous scientific and regulatory assessment, and authorization. This resource aims to help practitioners and patients to better understand what is on the market for cell and gene therapies. This report ultimately allows practitioners and patients to better make informed decisions, avoiding exposure to unproven and unlicensed cell interventions not approved by a regulatory or medical agency.
ISCT is a member of the National Academies of Sciences Engineering Medicine’s Forum on Regenerative Medicine. The forum brings together academia, industry, government, patient and provider organizations, regulatory bodies, foundations, societies, associations, and other groups, to discuss the challenges and opportunities of regenerative medicine, potentially improving the health of millions of people worldwide through the development of effective new therapies.
ISCT representation on this reputable forum has gone from two members to five over the past year.
Representing us on the forum are: Dr. Daniel Weiss, Karen Nichols, Dr. Catherine Bollard, Dr. Bruce Levine, and Dr. Patrick Hanley.
2019 ISCT Career Achievement Award Winner
Past Senior Editor, Cytotherapy
Co-Founder, ISCT-ASTCT Cell Therapy Training Course
Adventure and Achievement
Celebrating some of the World’s Top 100 Medicine Makers in 2019
Celebrating
Dr. Bruce Levine and Dr. Carl June
Business Captain
Dr. Claudia Zylberberg
Champion of Change
Dr. Massimo Dominici
ISCT Awards 2019
Recognizing the Achievements and Contributions of Our Membership
Acknowledging Adventure and Achievement
“In 1970, I was involved with my first bone marrow transplant which was a highly experimental procedure at the time. It was the excitement of seeing the success of a transplant in a child with immune deficiency that really got me started, and I’ve been in the field ever since.”
To commemorate his lifelong efforts, ISCT’s most prestigious award, the Career Achievement Award, was awarded during the 2019 Annual Meeting to John Barrett, MD, to recognize his service to the advancement of the field of cell and gene therapy at large, and to the society through his 17 years of dedicated contribution as Senior Editor to Cytotherapy, and his visionary role as co-founder of the ISCT-ASTCT Cell Therapy Training Course.
Dr. John Barrett has worked to advance the field of cell and gene therapy since the earliest parts of his career. The conclusion of his studies saw him shortly after within the UK hospital system, developing and delivering experimental treatments. Notably, Dr. Barrett’s work with bone marrow transplants at the time yielded significant advances in addressing immune system complications and donor rejection within clinical procedures. His successes in the hospital would see him nominated to work with the NHLBI in the United States in 1993. From that point forward, his work would take him into the cutting edge of cell therapy, advancing research in the areas of hematology and stem cell transplantation especially.
John has been with us at the ISCT for the long haul. In 2001, he published his first editorial as co-editor of Cytotherapy, then journal for ISHAGE, which would eventually evolve into the ISCT. From there, he served as editor-in-chief to the journal between 2002 – 2019. Dr. Barrett has experienced the evolution of the field, of the journal, and of the society throughout his years of service.
He has also demonstrated a commitment to giving back through training and mentorship. John is one of two co-founders of ISTC-ASTCT’s biennial Cell Therapy Training Course (CTTC), and has offered his time as co-director of the course since. CTTC brings together leading experts in cell and gene therapy with rising stars who are poised to conduct and deliver leading edge research that advances the field.
John is both a respected scientific investigator, and a successful scientific communicator. His work, spanning from over 350 original scientific articles and 150 reviews/editorials/book reviews, to his representation on numerous societies and their boards, and his tireless efforts to pass on his expertise by personally delivering training, and by overseeing programs like the CTTC, speak to his lifelong commitment to the task of clinical translation: the driving mission of the ISCT.
We are honored to have been given so many years of service by Dr. John Barrett, and are proud to acknowledge his lifelong efforts to give of himself to the field, to the society, and to the betterment of healthcare outcomes for patients on a global scale.
A globally leading publication on drug development, The Medicine Maker, distinguished several notable ISCT members on its 2019 Power List.
Categories of the Power List include Masters of the Bench, Industry Influencers, Business Captains and Champions of Change.
Those featured on the list conduct life-saving work in the global biopharmaceutical industry.
These are the ISCT members who were featured in this year’s power list.
Master of the Bench
Dr. Bruce Levine
President-Elect 2018-2020
International Society for Cell and Gene Therapy
Master of the Bench
Dr. Carl June
Richard W. Vague Professor in Immunotherapy
University of Pennsylvania
Business Captain
Dr. Claudia Zylberberg
Co-Founder, Chief Executive Officer and President
Akron Biotechnology
Champion of Change
Dr. Massimo Dominici
Past President (2014-2016), and President’s Task Force Chair
International Society for Cell and Gene Therapy
A globally leading publication on drug development, The Medicine Maker, distinguished several notable ISCT members on its 2019 Power List.
Categories of the Power List include Masters of the Bench, Industry Influencers, Business Captains and Champions of Change.
Those featured on the list conduct life-saving work in the global biopharmaceutical industry.
These are the ISCT members who were featured in this year’s power list.
Master of the Bench
Dr. Bruce Levine
President-Elect 2018-2020
International Society for Cell and Gene Therapy
Master of the Bench
Dr. Carl June
Richard W. Vague Professor in Immunotherapy
University of Pennsylvania
Business Captain
Dr. Claudia Zylberberg
Co-Founder, Chief Executive Officer and President
Akron Biotechnology
Champion of Change
Dr. Massimo Dominici
Past President (2014-2016), and President’s Task Force Chair
International Society for Cell and Gene Therapy
AWARDS AT ISCT Melbourne 2019
To recognize and help up-and-coming scholars in the cell and gene therapy field, the Society presents the ISCT Abstract Awards each year, awarding thousands of dollars to emerging scholars and an opportunity to showcase their research on a global platform. Please meet the 2019 winners:

Top Scoring Abstract ($1,500)
Yohei Sato, MD, PhD (Japan)

Best Poster Abstract ($1,500)
Lorena Braid, PhD (Canada)
Early Stage Professionals Abstract Award ($1,000)

Sophia Schreiber, MSc (Germany)

David Bishop, BMedSc(Hons), MBBS, FRACP, FRCPA (Australia)
Emerging Economy Abstract ($1,000)
ISCT 2018 Technologist Abstract Award ($1,000)

Sharon Ng Wah Suet, MSc (China)
Ultrastructure of Cytokine Induced Human Natural Killer Cells
AWARDS AT ISCT nA 2019 – MADISON, WI

Technologist Abstract Award ($500)
Todd Maguire, BSc (USA)
Abstract:
Validation of Cesca CAR-TXpress™ system for Activation in Scale-up CAR T cell Production

Early Stage Professional Abstract Award ($500)
Jayeeta Giri, PhD (USA)
Abstract:
Mesenchymal Stromal Cells Derived CCL2 and CXCL12 Promote Intestinal Homeostasis Through the Induction of Anti-Inflammatory M2 Macrophage Polarization
AWARDS AT SCSS-ISCT JOINT SYMPOSIUM
(Complimentary Main Conference Registration – ISCT 2020)

Best Short Talk
Esmond Lee, BE, PhD Student (USA)
Abstract: FOXP3 Gene Editing towards autologous stem cell therapy to cure IPEX Syndrome

SCSS-ISCT Joint Symposium Abstract Award
Raymond Smith, PhD (Singapore)
Abstract: IMB A*STAR: A synthetic heparan sulphate mimetic for enhancing BMP-2-mediated osteogenesis
AWARDS AT ISCT ANZ-ASSCR-AGCTS JOINT SCIENTIFIC MEETING
(Complimentary Registration – ISCT ANZ-ASSCR-AGCTS Joint Scientific Meeting)
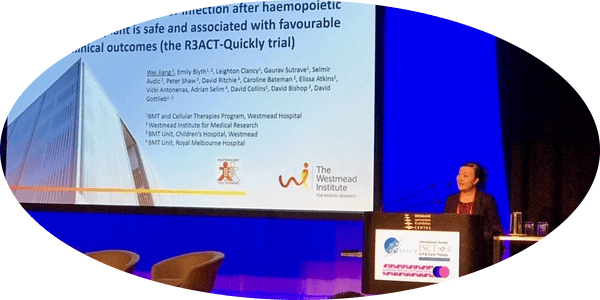
ISCT ANZ Early Stage Professional (ESP) Travel Award
Wei Jiang, PhD
Abstract: Rapid administration of third-party cytomegalovirus (CMV), Epstein-Barr virus (EBV) or adenovirus (Adv) specific T-cells (VSTs) post-haemopoietic stem cell transplant (HSCT)
Cytotherapy, the official journal for the International Society for Cell and Gene Therapy (ISCT), publishes novel and innovative results from high quality scientific and clinical studies in the fields of cell and gene therapy.
Across 2019, the journal, undergoing its 20th year of consistent and leading edge science, underwent a series of re-branding initiatives. From honing its focus and scope and transitioning to a wider editorial team to focusing on engaging ISCT membership and undergoing a visual overhaul, Cytotherapy has put itself in a position to advance and expand the field of Cell and Gene Therapy for clinical researchers, oncologists, hematologists, physicians, and regulatory experts alike. The highest impact factor of the journal to date was also reported in 2019, at a strong 4.293 for 2018.
Take a look at our Talking with Giants series, launched in 2019, which showcases leaders in the field of cell and gene therapy through informal question and answer articles. Talking with Giants is featured at the start of each issue of Cytotherapy.
You can read issues of Cytotherapy here.
Available to ISCT members and the cell and gene therapy community around the world, Telegraft is the Society’s bi-monthly e-newsletter and your go-to resource to keep up to date with the latest Society activities and important developments in cell and gene therapy. Led by Nancy Collins, the Telegraft Editorial Board compiles robust issues aiming to enhance communication and understanding between all Society constituencies: academic, clinical, basic and translational science, regulatory, technical, and commercial. In 2019 Telegraft strengthened its connection with Cytotherapy co-publishing the ISCT Talking With Giants series. Telegraft introduced other new features including “From the Blogsphere and Twitterverse” as well as features on ISCT members and leadership and the outstanding work they are doing.
You can read issues of Telegraft here.
Join a Truly Global Community Advancing Cell and Gene Therapy
ISCT membership benefits your cell and gene therapy career in a number of valuable ways with exclusive access and complimentary subscriptions to Telegraft and Cytotherapy, as well as networking opportunities to collaborate with key opinion leaders, government regulators, commercial partners and cell therapy technologists. An ISCT membership also provides:
- Reduced rates at ISCT-sponsored events, including webinars, technical workshops, research and clinical symposia, and the ISCT meetings.
- Access to members-only web resources, including the ISCT Member Networking Database and online discussion boards.
To learn more about ISCT memberships and how to join, visit our website.
Meetings – Save The Date
Our Continued Commitment
As the leading global Society focused on the translation of cell and gene therapy, our mission remains as important as ever in the face of these uncertain times.
We will deliver leading-edge science and connect you with the global ISCT community on the most innovative virtual conferencing platform available.
The Newly Updated ISCT Career Center
Recruiting, or looking to be recruited?
Visit the new ISCT Career Center. As part of our 2019 vision, we have restructured our job posting process to engage and to help expand laboratories worldwide as they seek qualified technicians, scientists, and business professionals. If you manage or are part of a lab, please inquire about our efforts to support your growth through catered job postings and outreach.
Our Volunteer Center
The ISCT Volunteer Center is the new hub designed to facilitate members seeking to join one of our 41+ standing committees, or to contribute to our bimonthly e-newsletter, Telegraft.
If you have a passion for the advancement of the field of cell and gene therapy, and you wish to contribute your voice towards shaping the field by sharing your expertise and experience, please check in regularly to the volunteer center, as opportunities are expected to expand.
Our Committees
ISCT has 41 stakeholder committees, working in a number of scientific, commercial and regulatory disciplines within cell and gene therapy. Joining an ISCT committee is an invaluable networking opportunity, allowing you to connect with cell and gene therapy professionals from all over the world. If you would like to bolster your career by joining an ISCT committee, visit our Committees page to see how.
Showcase Your Work:
 Cytotherapy
Cytotherapy
The official journal of ISCT (International Society for Cell and Gene Therapy) publishes novel and innovative results from high-quality scientific and clinical studies in the fields of cell and gene therapy. Reaching a global audience of cell and gene therapy professionals each month, content published in Cytotherapy is an essential and reliable resource for clinical researchers, oncologists, hematologists, physicians, and regulatory experts around the world.
Aims and Scope:
Studies evaluating the potency of experimental cell and gene therapies in clinically relevant animal models of disease and describing important advances in cell/gene-based product manufacturing and validation are welcomed. Results of clinical studies evaluating the safety and efficacy of cell and gene therapies in early and late phase trails are also of interest. In addition to Short Reports and Full-Length Articles, the journal also accepts Editorials addressing emerging trends and potential controversies in the field, and Review articles summarizing bodies of work that have made lasting impacts in the field.
Journal Format:
Cytotherapy accepts four categories of submissions (Please visit Cytotherapy’s journal submission page for more information):
Full-length article
| Short Reports
| Editorials
| Review articles
|
Telegraft
Available to ISCT members around the world, Telegraft is the Society’s leading global cell therapy newsletter which updates readers on new cell and gene therapy developments, regulatory updates, and regular columns on related meetings, organizations, and events, summaries of work being done in both academic and industry labs around the world.
If you are interested in contributing an article to Telegraft, or in helping out with our editorial process, please visit here.
Tools for Learning
ISCT has a large number of valuable and comprehensive resources for ISCT members and cell and gene therapy professionals. This toolbox of resources includes a useful glossary of cell therapy terms, the ISCT Cell Therapy Bioprocessing Tools and Reagents Database, a centralized catalogue of available products for use in cell therapy processing, manufacturing, and research, as well as valuable patient resources from the innovative ISCT Presidential Task Force (PTF) on the Use of Unproven and/or Unethical Cell and Gene Therapies website, which was launched in 2018.
Visit the Cell Therapy Community Resources page to learn more.