
JACIE 20th Anniversary Recognition Award
Presented to Riccardo Saccardi, JACIE Medical Officer

Catherine Bollard, MBChB, MD
Outgoing ISCT President
(May 2016 – May 2018)
USA
“During my tenure as president, I learned that ISCT’s strength is rooted in the diversity of its passionate members and staff.”
As an internationally focused society, we draw on the expertise and enthusiasm of those working in cell and gene therapy across the globe. Therefore, it is critical for the field, as a whole, that we are an international society. The cell and gene therapy Industry is growing globally and as the leading cell therapy society in the field, we must evolve with that growth. From around the world, ISCT brings together minds from academia, industry, regulatory bodies and many other groups in a meaningful way, allowing the industry to “connect and collaborate.”
It’s critically important that ISCT serves different regions across the globe and continues to serve more countries and regions developing cell and gene therapies. Our slogan, “Connect. Communicate. Translate.”, is appropriate because that is exactly what ISCT does, connecting and engaging a diverse global network of cell and gene therapy professionals so they can produce new, safe therapies for you and me.
2018 was also the year my presidency at ISCT sadly ended in May. My memorable time as president was highly enriching, both professionally and personally. I have since passed the torch onto Dr. John Rasko, who I am sure will serve the society very well during this time of growth in the field. As the Immediate Past President, I am still very much involved with the Society and I am extremely confident that ISCT will continue to flourish during this extraordinarily exciting trajectory for the cell and gene therapy field.

John EJ Rasko, AO, MBBS, PhD,
FRCPA, FRACP, FAHMS
ISCT President (2018-2020)
Australia
“In 2018, there was such pinch-yourself excitement in the industry. It was excitement stemming from the fact that after decades of hard work, cell and gene therapies have become a clinical reality.”
I am truly delighted to welcome you to ISCT’s very first annual report. This marks an important milestone in our Society’s history, which mirrors the maturation of our field of cell and gene therapy. Just as a cornerstone is often ceremoniously placed near the base of a building to record historical information, so our annual report will serve as an important foundational document for future reference. The report also provides further confirmation of ISCT’s commitment during my term to promote internal and external communication for the benefit of members and professional colleagues.
I will forever remember 2018 as a significant consolidation year with an increasing number of proven cell and gene-based medicines being approved in diverse markets worldwide. New innovative technologies, increasing investment, maturing regulatory approaches and ground-breaking research are cause for reflection and celebration. The initial glow of the realization that cell and gene therapy had arrived, was reinforced by the heart-warming reality of implementing these life-saving strategies.
In 2018, there was such pinch-yourself excitement in the industry. It was excitement stemming from the fact that after decades of hard work, cell and gene therapies have become a clinical reality. Cell and gene-based therapies to treat cancer, particularly for leukemia and lymphoma, and a few select genetic diseases are now available to patients in many regions.
As you read further in this report, you will learn more about the impact ISCT has made on the cell, gene and regenerative medicine fields. You will learn about all of the passionate people within the global community of ISCT, making a significant difference in the field, potentially helping millions of people around the world.
ISCT represents an international covenant dedicated to helping the public access the safest cell and gene therapies for themselves and their families. We all share common values. All human beings have a fundamental right to access excellent health care. ISCT’s mission, vision and values focus on ultimately improving human health and reduce suffering. It is not an easy mission, but is worth the hard work.
No matter where in the world they may be located, every ISCT member sings from the same songbook when it comes our common commitment of improving lives around the world. Because of that, I think ISCT is a shining light as we pursue our goal to alleviate suffering in those individuals with unmet medical needs.
As we move forward, growing and evolving, there is much more opportunity for ISCT to connect, communicate and translate through the talented membership of our Society. 2018 was a big year and I look forward to what the future holds for cell and gene therapy. Earlier, I likened our annual report to a building cornerstone. Often these grand blocks contain a ‘time capsule’ so that historians might learn more about the epoch in which they were set. Let us ensure that our field continues to flourish with safe and effective medicines based on cell and gene technologies. I hope that future generations might confirm the sense that we are indeed living at the beginning of a Golden Age of cell and gene therapy.

Queenie Jang, BSc (Pharmacy), MBA,
Chief Executive Officer
Canada
Before this evolution ever began, our founders started building the basis for this innovative field in 1992 as the only international society to bring together and to connect the entire translational value chain, helping build the thriving CGT field we know today.
Originally a society for hematotherapy and graft engineering, ISCT has since evolved, expanding its scope – and logo – to cell- and gene-based therapies, providing a wealth of education, tools, and resources to this growing field.
For 26 years, ISCT has orchestrated the work of thousands of clinicians, regulators, technologists, industry partners and academia to advance cell, gene and regenerative medicine for the benefit of patients worldwide. This includes the exciting approvals of CAR T-cell therapies which have made a huge impact – life-changing results – for patients around the globe. As more innovative cell and gene therapies come to market, ISCT recognizes the need to train the next generation of cell and gene therapy professionals to ensure the sustainability of the field.
“We are in the midst of a CGT revolution and immense growth for both ISCT and the cell, gene and regenerative medicine field at large.”
From our hematotherapy origins to our expanded cell and gene therapy scope, ISCT has a proven track record of evolving to meet the needs of an ever-changing industry. Join us, as we continue to connect, communicate and translate in establishing the CGT field as the next mainstream therapeutic area.




Dr. Daniel Weiss, MD, PhD
Chief Scientific Officer, ISCT
USA
“By working with researchers and the scientific community, we can safely bring these cell and gene therapies to market. Science underscores everything. It’s the foundation on which all of these therapies are based on – without the science, you have nothing.”
Academic and scientific research are the infrastructure of cell and gene therapies. Without visionary scientists and researchers dreaming up the next cell and gene therapy, these medicines would not exist.
“ISCT is one of the few organizations in the world that is supporting and facilitating the cell and gene therapy field,” Dr. Weiss says. “It is extremely gratifying to be involved with this pioneering work, possibly helping many people who are ill.”
He says that through collaborative scientific and academic research, ISCT members and the global cell and gene therapy field are able to communicate in one, universal language.
“Science is a global language,” he explains. “No matter what country you’re from, ISCT enables members from across the globe to collaborate on the academic and scientific aspects of cell and gene therapy. This is where the ideas for therapies are born.”
The University of Vermont professor says cell and gene therapy is a “very exciting field.”
“It’s an exciting, fast-moving, cutting-edge field and couldn’t see myself doing anything else,” he says with a smile. “I love it because it encompasses a number of different disciplines like cell therapy, gene therapy and cancer research.”
In response to a controversial article printed in Nature International Journal of Science, ISCT members published a response, applauding the article’s effort to discuss the vexing issue of stem cell tourism. The response made it clear that ISCT, a leader in the cell and gene therapy field, is against unethical and illegal selling of unproven stem-cell therapies of all ilk and mesenchymal stromal cells (MSCs) in particular. The ISCT authors also proposed that MSC terminology promotes deceit by medical tourism groups by specifically appropriating the key word “stem” as a means to confuse the public, calling back to ISCT’s landmark position statement on MSC nomenclature .
 | ISCT is represented on the International Organization for Standardization (ISO) TC 276 Biotechnology technical committee by ISCT MSC Committee member Dr. Sowmya Viswanathan. ISO TC 276 ScopeStandardization in the field of biotechnology processes includes the following topics:
Dr. Viswanathan and ISO/TC 276 Biotechnology works closely with related ISCT committees to identify standardization needs and gaps, and collaborate with other organizations to avoid duplications and overlapping standardization activities. For more information, visit the ISO/TC 276 page here. |
In 2018, ISCT scientific committees published four papers outlining innovative ideas and work taking place in cell and gene therapy.

Chair: Jacques Galipeau, MD, USA
Co-Chairs: George Muschler, MD, USA and Christian Jorgensen, MD, PhD, France
Co-Chairs: Rachele Ciccocioppo, MD, Italy and Giuseppe Orlando, MD, PhD, USA
| The ISCT Scientific Signature Series offers the unique opportunity to gather key opinion leaders in the chosen field to discuss, present and develop position statements to move the field forward. These events are aimed at driving thought leadership by providing content and building collaboration around concept papers, consensus statements, clinical networks, regulatory proposals, and recommendations for investment in clinical and basic research in cell and gene therapy medicine. The last series was in held in Florence as part of the regional meeting for Europe. |   |

Karen Nichols, Esq.
Chief Regulatory Officer, ISCT
USA
“It’s important to focus on this area because the regulatory process is what helps get safe and approved drugs into the market. Just like the academic and scientific sides, regulations are an essential step of approving safe therapies.”
“It began before the commercialization of CAR T therapies and was validated by their approval,” she explains. “These therapies are on the market and now helping many people who are stricken by disease from around the world.”
To help more people and to advance the clinical translation of cell and gene therapies, ISCT has a strong regulatory focus, driven by its Legal and Regulatory Affairs (LRA) Committees in North America, Europe, and Australia and New Zealand. These committees address challenges through liaison meetings with regulatory agencies, provide expert recommendations on official guidance documents, and deliver timely information and education to stakeholders. As a global strategy, the Global Regulatory Task Force aims to provide strategic oversight and support for the regional LRAs and the flagship Global Regulatory Perspective Workshop at the annual meeting, which is a unique forum that brings together regulators from around the globe.
“It’s important to focus on this area because the regulatory process is what helps get safe and approved drugs into the market,” she says. “Just like the academic and scientific sides, regulations are an essential step of approving safe therapies”.
But with this progress, there are significant challenges, particularly around training enough professionals to keep the industry’s momentum going. With more therapies being developed and regulated, there is an increased need for passionate people to work in the industry.
“I think there are hiring challenges depending on the geography we’re working in,” she says. “ISCT’s role is to, in my case, engage regulatory professionals from other societies. We need to collaborate with other groups to address the regulatory aspects of cell and gene therapy.”
“We need to be training and attracting younger generations, without a doubt,” she continues. “Not only will there be jobs for younger generations, but we need their skills and fresh insight to keep the industry going. I think a solution might be in the ESP program. A lot of heart and soul went into the program and folks are very passionate about preparing young generations to enter this workforce.”
In addition to the human resource issues, Karen says it is also a challenge working with the different regulatory frameworks for cell and gene therapies around the world.
“There are different frameworks we have to face in each country,” she says. “There’s also the varying maturity of those frameworks in different countries – some have very young, undefined regulatory structures, whereas others are more mature and more defined. This increases or decreases the level of uncertainty in getting approvals for these products.”
As ISCT’s Chief Regulatory Officer, Karen says she enjoys working with other sectors in the CGT industry – all for a common goal.
Since 2004, ISCT has been the host organization leading over 20 invited stakeholder organizations in an annual face-to-face meeting with the FDA Cell Therapy Liaison Meeting (CTLM). These closed meetings enable the cell and gene therapy community, and industry to inform the FDA of specific concerns, challenges and recent developments to advance the regulatory field.
The 2018 CTLM was held on January 30th in Bethesda, USA.
Since 2015, ISCT participates in bilateral meetings with the Health Canada BGTD and national stakeholder organizations in Canada in an effort to improve interactions between health care and the cell and gene therapy community as well as to improve the navigation of regulations and discuss challenges in the field.
The 2018 meetings were held on April 24th and December 4th in Ottawa, Canada.

The Quality and Operations Track is a series of sessions held at the ISCT Annual Scientific Meeting that are dedicated to educating technical and quality professionals in the cell and gene therapy field. At the 2018 ISCT Annual Scientific Meeting in Montreal, attendees of the track were educated on a number of timely topics including advancing standards for regenerative medicines as well as day-to-day operations in a stem cell lab.

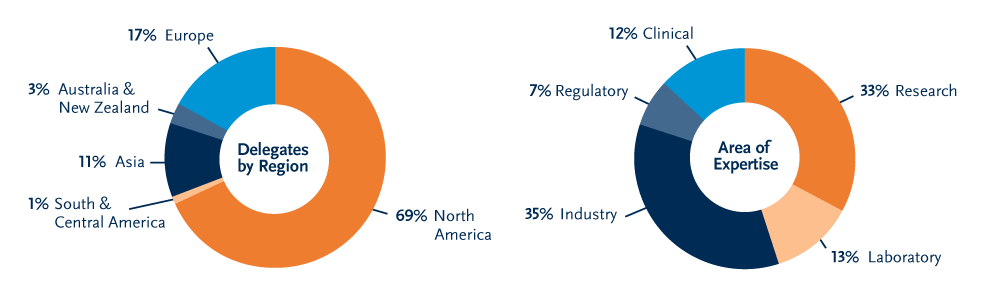
ISCT is a truly global organization with five distinct regions: North America, Europe, Asia, Australia and New Zealand, and South and Central America.

Membership: 51% outside of the United States from 60+ countries
With education as the core of ISCT’s value proposition to our members and the cell and gene therapy community, ISCT delivers highly curated scientific programming around the globe through its annual and regional meetings. The rotation of these meetings throughout the world provides its members with leading-edge education and networking opportunities to advance the field and drive the clinical translation of cell and gene therapies to patients.
ISCT works globally with organizations that share a common vision to drive the translation of cell and gene therapies for the benefit of patients worldwide.
Since 1992, ISCT has had connected more than 17,000 delegates through our Annual Meetings and communicated with more than 30,000 cell and gene therapy professionals at cutting-edge meetings, events, webinars and seminars to translate the advancement of research into clinical adoption and standard of care over the past 26 years.
Every year, the ISCT Annual Meeting delivers a highly curated scientific program showcasing the latest clinical results and technologies expanding global access to new and emerging cell and gene therapies.



To complement the globally focused Annual Scientific Meeting, ISCT hosts yearly regional meetings for the local members and industry partners.
ISCT provided global expertise at over 10 joint sessions with leading organizations including the Japan Society for Hematology (JSH), Asian Cellular Therapy Organization (ACTO), Associação Brasileira de Terapia Celular (ABTCel), Society for Immunotherapy of Cancer (SITC), the British Society for Gene and Cell Therapy (BSGCT) and Phacilitate Trade Mission (Japan and Korea), Business of Regenerative Medicine in Philadelphia, Stanford CGMP Symposium.
To address patient uncertainty around cell and gene therapy treatments throughout the explosive growth of the field, ISCT’s Presidential Task Force (PTF) on the Use of Unproven and/or Unethical Cell and Gene Therapies works to provide reliable and verified information to a global public.
Since its inception, the PTF has consolidated several up-to-date resources for the public regarding the field of cell and gene therapy:
Throughout this past year, the PTF took action on several issues around unproven cell therapy treatments and procedures. Chief among these was the issue of cell banking, on which the PTF issued a Statement of Concern on October 21st. Following this, the PTF took action on November 13th, creating a consortium of professional and educational societies to provide oversight and to protect the development of legitimate cell therapy products so as to preserve the public’s trust in this growing field.
Finally, on March 27th, members of the ISCT PTF co-authored a publication critically discussing the current status of cell therapies for lung disease, “Translating Basic Research into Safe and Effective Cell-based Treatments for Respiratory Diseases “
This global guide addresses the issue of unproven cell and gene therapies, which are currently being advertised, sold and administered to patients, although they fail to achieve recognized medical standards of proof for safety or efficacy. These unproven treatments are often expensive and offered outside the coverage of routine clinical care. They are not part of a conventional clinical trial and lack regulatory oversight. With this document, the ISCT Presidential Task Force hopes to educate patients and health care professionals, providing resources to determine whether a therapy has been deemed safe and effective by the appropriate governing bodies.
The ISCT CGT Market Authorization Report is a regularly updated document that will be republished yearly and available online.
This report is the result of an increase in the number of CGT market authorizations, as well as an increase in unproven approaches where cells are delivered as treatments without rigorous scientific and regulatory assessment, and authorization. This resource aims to help practitioners and patients to better understand what is on the market for cell and gene therapies. This report ultimately allows practitioners and patients to better make informed decisions, avoiding exposure to unproven and unlicensed cell interventions not approved by a regulatory or medical agency.
ISCT is a member of the National Academies of Sciences Engineering Medicine’s Forum on Regenerative Medicine. The forum brings together academia, industry, government, patient and provider organizations, regulatory bodies, foundations, societies, associations, and other groups, to discuss the challenges and opportunities of regenerative medicine, potentially improving the health of millions of people worldwide through the development of effective new therapies. The ISCT representatives on this reputable forum are Dr. Daniel Weiss and Karen Nichols.

Bill Milligan, Co-Chair, ISCT Business Models and Investment Sub-Committee
Patrick Rivers, Co-Chair, ISCT Business Models and Investment Sub-Committee
There is no doubt that the cell and gene therapy world is experiencing unprecedented growth at the moment. While it is an exciting time for cell and gene therapies (CGT), it is also a new, evolving field with a significant need for knowledgeable guidance and education. To support this need for providing more knowledge and education, as it relates to financing CGT research and development, ISCT created the investigators to investors (“i to i”) Program.
“The program will help enable investigators to better understand how to present a compelling case to investors to secure support for their technology or product candidate development,”
explains Bill Milligan, Co-Chair of ISCT’s Business Models and Investment Sub-Committee. “The investigator needs to really understand and articulate how their CGT candidate works, how to make it, and what benefit it is going to bring the patient. On the other side, we can educate investors about the important considerations for investing in this new CGT industry. It’s a win-win situation.”
Patrick Rivers is a Co-Chair of the sub-committee and he is also a Managing Principal at Aquilo Capital Management. He emphasizes that as an investor, the i to i Program is a valuable resource that is needed in the industry right now.
“Cell and gene therapy is a complex and highly technical space within drug development,” Patrick explains. “One of the goals of the i to i Program is to provide investors with a forum to learn more about the challenges and opportunities in this rapidly growing landscape within human therapeutics. Ultimately, we want to make it easier for investors to execute scientific diligence that gets them comfortable with investing more capital in cell and gene therapy companies.”
Unlike conventional medicines, cell and gene therapy development has many more complex factors to consider, including intricate manufacturing dynamics which result in higher costs, and advanced cold-chain infrastructures for storage and distribution.
“The whole world is starting to see how much benefit there is in these emerging cell and gene therapies, but also how complicated it is,” said Bill, who is also Senior VP of Business Development at Steminent BioTherapeutics Inc..
To determine further what financial backers are looking for from investigators, ISCT partnered with Bloomberg Intelligence to develop and distribute a survey last fall to over 2,000 investors. The information gathered from the survey is being used to create educational initiatives to help empower investigators in the field.
“With the i to i Program, we want to take the elements we learned from the survey and turn that into educational, insightful information for investigators,” Bill commented.
Patrick says the information from the survey is valuable to investors as well.
“By first exploring the current attitudes and concerns of investors regarding cell and gene therapy with this survey, we’ve identified several areas where we can build content using the thought leadership within ISCT, directing that content back to the investment community,” Patrick commented.
“Investors are always looking for new avenues to conduct diligence that gives them an informational advantage relative to other market participants – the i to i Program can help them achieve this for CGT.”

With the dramatic growth in the cell and gene therapy field, there is an urgent need to train the next generation of professionals. ISCT is playing a key role in developing the careers of Early Stage Professionals (ESP) as they enter the CGT field in record numbers.
“The biggest problem facing the growth around the world is a shortage of qualified people – it is a global, industry-wide issue.”
– Bruce Levine, PhD, President-Elect, ISCT
Over the past 10 years, the cell and gene, and regenerative therapy field has been experiencing unprecedented growth around the world. The US Food and Drug Administration predicts that, incredibly, the approval of cell and gene therapies will increase up to 900 percent in the next 10 years.
Long-time ISCT member and President-Elect Bruce Levine believes the field is going through a very pivotal phase at the moment.
“I think that we have a huge amount of seed and young plants spread around and there is going to be a weeding-out process,” he says. “We’re all going to grow and some are going to be successful therapies and companies, but others may not. The ones given sunshine and water and sustenance will grow and flourish – the ones in the shade without water will die away. We’re in a very unique phase of innovation where there are there are many new ideas coming out from academia now being developed by industry – the way to effective therapies is through different approaches.”
Even though times are positive in the cell and gene therapy field, there are challenges, particularly with the qualified human resources to accommodate this boom in the cell and gene therapy field.
“The biggest problem facing the growth around the world is a shortage of qualified people – it is a global, industry-wide issue,” he says looking out the window. “The field is growing so fast that there just aren’t enough undergrad and grad students who have a background in cell and gene therapy. So we need to encourage that pipeline of talent while taking people from other fields and cross-training them.”
With this dramatic growth in the cell and gene therapy field, Bruce thinks that we need the qualified infrastructure in place to train future professionals in the relatively young cell and gene therapy industry.
“We need programs in place to train these people,” he says. “I’m part of a couple of programs at our institution and collaborators for education on cell manufacturing and training programs. Because it is such a young industry, we are just thinking about how expand and build on existing programs to train more cell and gene therapy professionals.”
In August 2018, the ESP Committee launched the ISCT Mentoring Program following a successful pilot program in 2017. Mentors and mentees represented all five ISCT regions. The program provides ESPs with a unique opportunity to receive professional guidance from leaders in the cell and gene therapy field. The program pairs an experienced cell and gene therapy leader with two mentees who learn and grow from the knowledge passed down by the mentor. Recruited from a range of cell and gene therapy-related areas, the experienced ISCT mentors provide a wealth of career and professional development guidance for aspiring cell and gene therapy professionals entering the field.
Australia and New Zealand
Europe
North America
South & Central America
The ISCT 2017-2019 Strategic Plan identified the importance of grooming the next generation of ISCT leaders. This led to the development of a strategic objective to have one Early Stage Professional (ESP) on every ISCT committee. This objective strengthens the future of the Society and the cell and gene therapy field by involving younger generations in ISCT’s many initiatives.
ISCT ESP Networking event at ISCT 2018 in Montreal, Canada
There is an unfulfilled need for cell therapy training covering the process of translational research to cell manufacturing and clinical trials in cellular therapy including regulatory components. As a result, ISCT and the American Society for Blood and Marrow Transplantation (ASBMT) have created the exclusive Cell Therapy Training Course to train the next generation of translational experts in cellular therapy. After a thorough and comprehensive application process, six North American scholars and six international scholars are selected to attend this leading-edge course, taught by world class faculty. The third biennial 2019 Cell Therapy Training Course will be held this fall in Philadelphia, hosted by the University of Pennsylvania.
With the tremendous growth in the cell and gene therapy field comes a greater need to attract emerging professionals to drive this thriving industry. ISCT created the Early Stage Professionals (ESPs) Committee to provide a networking and educational forum for new cell and gene therapy professionals from around the globe, working to establish the future leaders of not only ISCT, but the field at large. The ESP Committee is comprised of 20 members from 6 countries who develop programming targeted at their fellow young professionals including sessions at the ISCT Annual Meeting, mentoring programs, the Young Investigator Abstract Awards, and more.
“I knew I could make a difference if I chose this field.”
Dr. Nancy Collins’ passion for curing cancer came at a young age.
“It started when I was in eighth grade,” she recalls. “For my science project that year, I chose leukemia. I chose it because a friend of the family had died of leukemia and I was interested in why.”

“I looked around where the med tech worked and I thought he had a lot of cool stuff and was doing important work,” she recalls. “He explained to me how they diagnose leukemia, what a centrifuge was, what a white blood cell was and I was hooked – it lit a spark. I knew I could make a difference if I chose this field.”
40 years later, that spark has become a bright, burning flame. Nancy got her PhD in microbiology and immunology (“I became allergic to all of my lab animals.”) and has become a leading authority in cell and gene therapies, establishing a distinguished research career – and co-founding the ISCT.
As senior editor for ISCT’s newsletter, The Telegraft, as well as a number of other roles including teaching at the University of Toledo, Nancy has also become a mentor to future generations in the cell and gene therapy field, a job she says is “fulfilling,” especially after retirement.
“We need to transfer the existing knowledge and high standards that ISCT has built and transfer them to younger, enthusiastic generations so they can continue the good work,” says the winner of last year’s ISCT Career Achievement Award. “As editor of the Telegraft newsletter, I have the opportunity to mentor others and give back what I know. When I was offered the editor position as a mentoring opportunity it hit my sweet spot because if we set up a good mentoring system, we can pass a lot of knowledge to the younger generations.”
The cell and gene therapy field has recently experienced a period of explosive growth and because of that, Nancy says that organizations like ISCT are needed more now than ever.
“We need to have strong communications between the various parts of the field,” she explains. “The clinical part, the translational part, the laboratories, the regulatory bodies – they all need to be coordinated and we need everyone talking to each other. ISCT is the central forum that connects all of these groups and people together to create new therapies for people around the world.”
For 17 successful years, Dr. John Barrett devoted his time and seasoned knowledge as the Senior Editor of Cytotherapy, the official journal of ISCT.

As to success, the journal metrics speak for themselves. First, the volume of papers published has increased three-fold. In 2000 we had six issues per annum and published 41 papers. In the last 12 months we have published 26 reviews, commentaries, and 123 regular articles and letters. Meanwhile our impact factor began below 1.0 in 2000, rose to the 3’s in 2006, reaching 3.99 this year. The contents have broadened from the solid base of mesenchymal stromal cells to cover the development of a huge spectrum of cell types used in therapy, including iPS cells, and increasingly gene-modified cells, and the new field of exosomes. Notable growing areas in the journal repertoire are immunotherapy – T cells, NK cells and dendritic cells, clinical cell therapy trials, technical and regulatory reports and numerous position papers and authoritative documents from the ISCT membership.
At the heart of the success of the journal is the editorial team, the publisher, and the Society. Sustaining a journal that meets the needs of the authors and the acclaim of the readership has needed teamwork and strong collaboration between Elsevier, the publisher, the ISCT, and critically the Senior Editor and the Associate Editors.
Elsevier has served as our publisher since 2013 and continues to be a powerful force for delivering our product, driving expansion, and supporting the goals of the journal and the ISCT. In particular I should mention our past publisher Vindra Dass and Angela Welch our current publisher who serves the journal with imagination and dedication. Elsevier is strongly committed to fostering the journal’s upward trajectory. Our publishers can call upon the worldwide presence, sophisticated information technology and not least, the international recognition of Elsevier.
ISCT has always recognized the importance of Cytotherapy for the Society and Queenie Jang, our CEO, has been a major influence in the development and expansion of the journal, strengthening its integration within ISCT. Since 2000, the journal has lived through eight ISCT presidencies and the fact that four of them have served, or continue to serve as associate editors, is an indication of the close association of the journal with the ISCT.
The team: I have had the privilege of working with a team of associate editors who have put in hours of work to process manuscripts and provide their input on the quality or otherwise of the material submitted. Metrics of paper handling provide a feedback to every editor and have tracked an improvement in the pace of manuscript turn-around. We are conscious of our role in serving would-be authors by rapid paper processing, fair, expert, and in depth reviews, and a full explanation of the reasons for declining a manuscript when we reject. The rarity of author complaints is a sign that we provide good customer relations here. I cannot finish without listing all my colleagues who have served as editors from Graham Sharp, Gunnar Kvalheim, Jed Wolchok, Edwin Horwitz, Massimo Dominici, Robert Deans, Cath Bollard, Katy Rezvani, Don Phinney, John Rasko, to Peiman Hematti. To these loyal colleagues who have kept the lifeblood of Cytotherapy flowing, day in, day out, for many years – and to Elsevier and the ISCT – a heartfelt thank you! I could not have done it without you!
Cytotherapy is fortunate to have Donald Phinney as its new Senior Editor. A strong ISCT member with a broad experience in journal publishing, Don will handle the day-to-day running of the journal with rapid and impeccably fair and well considered turnarounds for submitted papers. Beyond that, he will innovate the journal’s shape and contents. I look forward to seeing Cytotherapy continue to rise and remain a flagship not just for the ISCT but the for the entirety of this fast-moving and always exciting cellular therapy field.

To help up-and-coming scholars in cell and gene therapy field, the Society presents the ISCT Abstract Awards, which awards thousands of dollars to emerging scholars and an opportunity to showcase their research on a global platform. Please meet the 2018 winners:
Abstract: Human Placental-Derived Adherent Stromal Cells Co-Induced with TNF-α and IFN-γ Inhibit Triple-Negative Breast Cancer in Nude Mouse Xenograft Models
Abstract: Allogeneic Amniotic Epithelial Cells for Established Bronchopulmonary Dysplasia in Premature, Low Birthweight Infants: A First-in-human Safety Trial

Presented to Riccardo Saccardi, JACIE Medical Officer

Abstract: Safety and Feasibility of a Tolerogenic Dendritic Cell-Based Vaccination in MS: A Collaborative Initiative Comparing Intranodal and Intradermal Cell Administration

Abstract: Targeted Stromal Cells Expressing Trail as a Therapy for Lung Cancer

Abstract: Combined Cell and Gene Therapy Towards the Treatment of Age-related Macular Degeneration and Diabetic Retinopathy

Abstract: Close Neighbors in the Niche: Paracrine Signals From Endothelial Cells Promote Alveolar Epithelial Differentiation of Induced Pluripotent Stem Cell-derived Lung Progenitors

Abstract: Safety of Stem Cell-Derived Encapsulated Liver Tissue to Treat Liver Failure: Immune-Isolation and Absence of Foreign Body Reaction or Tumor Formation Upon Transplantation

Presented to Riccardo Saccardi, JACIE Medical Officer

Abstract: Safety and Feasibility of a Tolerogenic Dendritic Cell-Based Vaccination in MS: A Collaborative Initiative Comparing Intranodal and Intradermal Cell Administration

Abstract: Targeted Stromal Cells Expressing Trail as a Therapy for Lung Cancer

Abstract: Combined Cell and Gene Therapy Towards the Treatment of Age-related Macular Degeneration and Diabetic Retinopathy

Abstract: Close Neighbors in the Niche: Paracrine Signals From Endothelial Cells Promote Alveolar Epithelial Differentiation of Induced Pluripotent Stem Cell-derived Lung Progenitors

Abstract: Safety of Stem Cell-Derived Encapsulated Liver Tissue to Treat Liver Failure: Immune-Isolation and Absence of Foreign Body Reaction or Tumor Formation Upon Transplantation

Abstract: First Trimester Human Umbilical Cord Perivascular Cells Expanded With CGMP Compliant Human Platelet Outperform Conventional MSC Sources As Regenerative Therapy in a Rat Model

Abstract: Safety Considerations in the Generation of Clinical Grade Autologous IPS Cell Lines

Abstract: Human Mesenchymal Stromal Cells Administered After Radiotherapy and Surgery in a Soft Tissue Sarcoma Mouse Xenograft Model Do Not Promote Local Recurrence or Metastasis

Abstract: Safety of Stem Cell-Derived Encapsulated Liver Tissue to Treat Liver Failure: Immune-Isolation and Absence of Foreign Body Reaction or Tumor Formation Upon Transplantation

Abstract: Close Neighbors in the Niche: Paracrine Signals From Endothelial Cells Promote Alveolar Epithelial Differentiation of Induced Pluripotent Stem Cell-derived Lung Progenitors

Abstract: Combined Cell and Gene Therapy Towards the Treatment of Age-related Macular Degeneration and Diabetic Retinopathy


2018 was a big year for Cytotherapy, the official journal for ISCT. An essential resource for cell and gene therapy clinical researchers, oncologists, hematologists, physicians, and regulatory experts, the publication named Dr. Donald Phinney as the new senior editor as well as Dr. Oscar Lee, Dr. Luis Ortiz and Dr. Sowmya Viswanathan as the new associate editors. The new editorial staff brings a wealth of experience to the journal, undoubtedly reinforcing Cytotherapy as a premier source for cutting-edge findings, clinical trials of cell-based therapies, and news and opinion pieces on all aspects of the rapidly expanding field of cell-based treatments for cancer, degenerative disorders, immunotherapy and stem cell transplantation.
You can read issues of Cytotherapy here.
Available to ISCT members around the world, Telegraft is the Society’s leading global cell therapy newsletter which updates readers on new cell and gene therapy developments, regulatory updates, and regular columns on related meetings, organizations, and events, summaries of work being done in both academic and industry labs around the world. In 2018, not only was Telegraft redesigned with a fresh new look, but the bi-monthly e-newsletter named Dr. Nancy Collins as its new senior editor, a prominent researcher, mentor and leader in the cell and gene therapy field. Telegraft also recruited two Early Stage Professionals (ESPs) Dr. Alireza Abazari and Dr. Satyam Arora, as the new junior associate editors, bringing fresh perspectives to the publication.
You can read issues of Telegraft here.
ISCT membership benefits your cell and gene therapy career in a number of valuable ways with exclusive access and complimentary subscriptions to Telegraft and Cytotherapy, as well as networking opportunities to collaborate with key opinion leaders, government regulators, commercial partners and cell therapy technologists. An ISCT membership also provides:
To learn more about ISCT memberships and how to join, visit our website.
ISCT has 41 stakeholder committees, working in a number of scientific, commercial and regulatory disciplines within cell and gene therapy. Joining an ISCT committee is an invaluable networking opportunity, allowing you to connect with cell and gene therapy professionals from all over the world. If you would like to bolster your career by joining an ISCT committee, visit our Committees page to see how.
Are you interested in recruiting for a cell therapy-related position in your company or institution? The ISCT Career Center is the perfect opportunity to highlight your organization, profile open positions, and recruit highly qualified cell therapy professionals from a targeted audience of cell therapy experts, including scientists, technologists, and business professionals.
ISCT has a large number of valuable and comprehensive resources for ISCT members and cell and gene therapy professionals. This toolbox of resources includes a useful glossary of cell therapy terms, the ISCT Cell Therapy Bioprocessing Tools and Reagents Database, a centralized catalogue of available products for use in cell therapy processing, manufacturing, and research, as well as valuable patient resources from the innovative ISCT Presidential Task Force (PTF) on the Use of Unproven and/or Unethical Cell and Gene Therapies website, which was launched in 2018.
Visit the Cell Therapy Community Resources page to learn more.
Cytotherapy, the official journal of ISCT (International Society for Cell and Gene Therapy), publishes novel and innovative results from high-quality scientific and clinical studies in the fields of cell and gene therapy. Reaching a global audience of cell and gene therapy professionals each month, content published in Cytotherapy is an essential and reliable resource for clinical researchers, oncologists, hematologists, physicians, and regulatory experts around the world.
Aims and Scope
Studies evaluating the potency of experimental cell and gene therapies in clinically relevant animal models of disease and describing important advances in cell/gene-based product manufacturing and validation are welcomed. Results of clinical studies evaluating the safety and efficacy of cell and gene therapies in early- and late-phase trials are also of interest. In addition to short reports and full-length articles, the journal also accepts editorials addressing emerging trends and potential controversies in the field, and review articles summarizing bodies of work that have made lasting impacts in the field.
Journal Format
– Editorial
– Reviews
– Short Reports
– Full-Length Articles on:
– Translational Studies
– Cell Therapy
– Immunotherapy
– Gene Therapy
– Regenerative Medicine
– Manufacturing
– Regulatory
Please visit the journal submission page for more information.

Queenie Jang, BSc (Pharmacy), MBA
Chief Executive Officer
Canada
Over the last year, ISCT has focused its investments on building a home for workforce development within the DNA of ISCT. Working with our expansive network of global key opinion leaders and subject matter experts, we have made significant investments to expand our educational offerings. From online resources to practical in-person programs, ISCT members now have access to a wide array of supports.
In recent years, the Cell and Gene Therapy (CGT) field has seen exponential growth, which has outpaced the rate at which new professionals enter. As result, we have witnessed major skill and labor shortages affecting the entirety of the field. Identifying this as a critical issue to the advancement of the sector, ISCT is motivated to be at the forefront of addressing this issue. We see it as our duty to Support the driven and diverse group of regulatory, commercial, and academic professionals and remove barriers and open doors for newcomers entering the field.
Through forming strategic partnerships with outside organizations such as CMaT ANd&AT and Universidad de Granda, ISCT has successfully developed and globally deployed a number of workforce programs spanning translational research to good manufacturing practices and regulatory understanding. 2022 marked not only the launch of the online workforce development in biomanufacturing course in partnership with CMAT but also the decision to make this a recurring program offered four times annually due to the critical need and demand for skilled personnel. Through our partnership with ANd&AT and Universidad de Granda, ISCT, was proud to awarded three European ISCT members full-tuition scholarships to enrol in the Master in Manufacturing of Advanced Therapy Medicinal Products (ATMPs).
Taking a step further to demonstrate ISCT’s unwavering commitment to helping bridge the labour gap in the market, alongside our dedicated head office liaisons, ISCT has formed a specialist workforce development committee. This committee, in collaboration with our global network of subject matter experts, will design training programs created by ISCT members for ISCT members that are tailored to the specific needs of professionals based on their region, area of focus and skill level.
Understanding the criticality of Early Stage Professionals (ESP) for the continued growth of the CGT sector, ISCT has made a deliberate decision to expand and invest in our ESP resources, programs and committees. ESPs currently make up a third of our overall global membership, and we are delighted to see these members becoming more active and engaged in the society. Our ESP Mentoring Program and our ESP Leadership Development Program reached record numbers of participants in 2022, allowing us to connect ESPs with our global CGT network and provide them with practical experience and connections that will last a lifetime. If the cell and gene sector is to continue to grow at the current rapid pace, we must continue to support ESPs and provide them with the tools they need to succeed and excel in their careers.
With workforce development being the greatest challenge that lies ahead for cell and gene therapies, ISCT is proud to champion and spearhead these initiatives. ISCT members, no matter what stage of their professional development, now have more access than ever to the key knowledge and networks that they need to become an effective part of the workforce. We will continue to work collaboratively with our all our stakeholders to develop paths and open doors for both interested professionals and newcomers alike.
Motivated to be a part of the solution and support the continued and sustainable growth of the cell and gene therapy sector, ISCT is spearheading efforts in workforce development, training, and education. In 2022 ISCT was a key player in driving the development and deployment of programs and initiatives available not only to ISCT members but also to the wider CGT community.
In 2022, ISCT successfully launched two new training programs to address critical skills gaps in manufacturing, partnering with the Andalusian Network for the Design and Translation of Advanced Therapies (ANd&tAT) to offer three full-tuition scholarships to ISCT members in Europe. While in parallel, partnering with CMaT, the NSF Engineering Research Center for Cell Manufacturing Technologies, to deliver the first edition of the online program for biomanufacturing.
Recognizing that a third of ISCT members are early-stage professionals, ISCT invested significant resources in expanding the global ESP mentoring and leadership development programs. For the wider global ISCT community, the society also made significant efforts to expand its digital resources for not only facilitating topical sessions but also encouraging cross-collaboration with regional committees.

Bruce Levine, PhD
ISCT Past President (2020 – 2022)
United States
“My passion for ISCT stems from the fact that in my career, no other society has played as integral a role in my professional development.“
Patrick Hanley
ISCT Representative to NASEM
Childrens National Hospital
United States


Meagan Pasternak, BA
ISCT Head Office
Canada

Elena Cooper, MSc
ISCT ESP Committee
United States

Maryam Pasdar, BSc
ISCT ESP Committee
United States
Being recognized as a leader in driving efforts in workforce development, ISCT was invited to take part in the workshop on Training the Regenerative Medicine Workforce for the Future hosted by the Forum on Regenerative Medicine at the National Academies of Sciences, Engineering and Medicine (NASEM).
The virtual workshop, Co-Chaired by ISCT North America Regional VP, Patrick Hanley, featured sessions from ISCT Head Office Senior Manager Meagan Pasternak and ISCT ESP Members Elana Cooper and Maryam Pasdar, which addressed issues related to lack of workforce diversity and opportunities to support workforce development in the future.
Identifying the limited number of skilled workers in manufacturing and product development as a major barrier to growth in the CGT sector, ISCT partnered with The National Science Foundation (NSF) Engineering Research Center for Cell Manufacturing Technologies (CMaT) to develop and deliver the first Workforce Development in Biomanufacturing course.
Developed by field experts from academic, regulatory, clinical, and commercial domains, the online program introduces key topics in cell and gene therapy manufacturing not only to up skill the existing biomanufacturing workforce but also provide those transitioning from other related fields with the fundamental knowledge and skillset unique to the space.
Following the successful global launch of the pilot program in June 2022, ISCT announced its plans to continue to offer the course on an annual basis and introduce three additional program dates throughout the year.
To further build on the learning of the online program, ISCT’s partnership with CMaT will see the introduction of the first innovative hands-on, on-site course, which will launch mid-2023.
Feedback from Inaugural Course

Queenie Jang,
BSc (Pharmacy), MBA
CEO, ISCT
Canada
“This biomanufacturing course, delivered in collaboration with CMaT, is a first step to address the broader needs of CGT training for both industry and academia. The overwhelmingly positive feedback we have received from the participants who took part in the course shows us that we are on the right track.”
“CMaT is tremendously excited to collaborate with ISCT and offer this critically needed course for the Cell Therapy Industry. We were humbled to see the community’s strong support and enthusiasm for this first offering”

Krishnendu Roy, PhD
Robert A. Milton Chair, CMaT
United States
“Working in the GMP facilities alongside experienced teachers and other international students enabled viable discussions, effective sharing of experiences and allowed us to put theory into practice.”

Katja Sirviö
MSc (Pharm)
Kuopio Center for
Gene and Cell Therapy
Finland

Alejandro Barquero
MPharm, MSc
Advent Bioservices Ltd
United Kingdom
“If you want to broaden your network and have fruitful exchanges with other field professionals from different ATMP backgrounds and countries, this is definitely the program for you.”
In partnership with ANd&AT and Universidad de Granada, supported by an educational grant from Miltenyi, ISCT was delighted to launch the first ISCT Master Scholarship Program in 2021/2022.
Available to ISCT members in Europe, the program offers three full-tuition scholarships to enroll in the Master Degree or Specialization Degree in Manufacturing of Advanced Therapy Medicinal Products, offered by the University of Granada/ Andalusian Network for the design and translation of Advanced Therapies (ANd&tAT).
The first of its kind, these programs are uniquely designed for technologies presently working in Good Manufacturing Practice (GMP)-compliant facilities within Europe producing cell therapy, gene therapy or tissue-engineered products for human use.
Following the demand for the 2021/2022 course, ISCT will continue to offer these scholarships on a biennial basis.
Congratulations to the awardees of the inaugural ISCT Europe Master Programme Scholarship on their completion of the 2021/2022 Master in Manufacturing of ATMPs at the University of Granada/ Andalusian Network for the design and translation of Advanced Therapies.
2021/2022 Scholars:
In 2022, ISCT was delighted to continue The ESP Mentoring Program. Designed to help guide the career and professional development of early-stage CGT professionals, the program connects ESPs with ISCT’s global network of CGT experts.
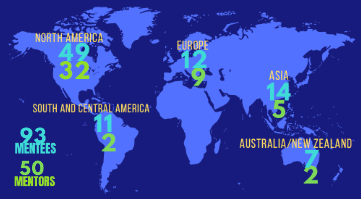
Since its launch in 2018, the mentoring program has seen significant growth with benefits for both mentee and mentor applications alike. 2022 was a stand out year for the program with 93 participating mentees and 50 participating mentors from across the globe.

Lindsay Davies, PhD
CellTherEx Consulting
Sweeden
“I have been a mentor for the last 2 years and really enjoyed speaking with ESPs, learning where they feel they need support and help for further development and tailoring sessions and topics to their individual requirements. I believe I have learnt as much from them as they have been able to learn from me.”
“If I were to describe in one word my early experience with my mentor, this word would be EMPOWERING! It’s exactly what I was hoping for. Early on they helped me identify my professional strengths and deficits, and helped me expand my research ideas.”

Diana Cirstea, MD
Massachusetts
General Hospital
United States
“I have had the opportunity to work with big names in the field, learn from them and apply the knowledge in my developing research. Since committee members are from around the world, I have gained exposure to global perspectives and updates on the latest methods and study directions“
Reza Yarani, PhD
Stanford University
United States
The ISCT ESP Leadership Development Program offers Early Stage Professionals (ESPs) a chance to join an ISCT Committee in a formal ESP position for a 2-year term providing a practical and immersive learning experience.
In 2022, ISCT was delighted to receive 46 applications from ESP members interested in the program. These applications were from over 18 countries globally, and positions were awarded on participating scientific, industry, regulatory, regional and communications committees.
Through the program, ESPs receive guidance from key global opinion leaders while collaborating on real-world projects in their area of interest that will advance the field of cell and gene therapy.
With a focus on expanding access to educational resources on a global level, ISCT leaders were motivated to develop and deliver a strong portfolio of webinars featuring both member-exclusive and open-access content. Many of the webinars were delivered as curated series leveraging ISCT expert committees to engage with international experts to share perspectives and knowledge with the wider community.
To foster global engagement and learning, ISCT encouraged cross-collaboration between regional committees to host joint webinar series, bringing together the ISCT North America and the ISCT South & Central America regional executive committees to deliver a series on Cancer Vaccines for Oncological Therapy and Implementing a new clinical CAR T program: Manufacturing your own.
2022 marked an unprecedented level of engagement for the ISCT webinar series, with members accessing content live and on demand.
ISCT has a specialized career site focused on CGT talent and opportunities. In 2022, the site saw record-breaking engagement. with the increasing demand for talent, ISCT is proud to provide a resource tool that presents quality opportunities to talent, ensuring that career seekers are able to use it as a reliable and selective point of access for first-choice job options in cell and gene therapy.
The career centre also provides job posters access to a select group of global CGT professionals with the expertise and skills needed to thrive in a fast-growing field to help streamline the recruitment process.

ISCT develops and delivers key expertise in regulatory and quality operations through the collaborations of several key stakeholder committees.
These include the Global Regulatory Task Force, which assesses and actions opportunities for regulatory harmonization and consultations, the regional legal and regulatory affairs committees, which closely track and advise on decisions from regional regulatory bodies, and the Lab Practices Committee, which focuses on the development of key member-facing resources that bolster the knowledge available to ISCT members.
Since 2004, ISCT has been the host coordinating over 15 stakeholder organizations in the annual FDA Cell Therapy Liaison Meeting (CTLM). These closed meetings enable the cell and gene therapy community to inform the FDA of specific concerns, challenges and recent developments to advance the regulatory field.
Each year, the CTLM report provides valuable coverage of key topics for regulatory consideration, summarizing the presentations that took place at the meeting, and providing key takeaways that are particularly useful to sector stakeholders.
The 2022 FDA CTLM focused on the following topics:
ISCT offers regulatory review and consultation from cell and gene therapy experts for regulatory authorities worldwide.
Review and recommendations are made by our Global Regulatory Task Force, as well as by regional Legal and Regulatory Affairs Committees in our North America, Europe, and Australia and New Zealand regions. Each consultation consists of significant comments, suggested revisions, and edits for regulatory documentation.
Below are the major guidance reviews in 2021:



Regulatory Consultations and Supports
Consultation with WHO: WHO Considerations for Regulatory Convergence of Cell and Gene Therapy Products (February 7 -9)
ISCT was invited by WHO to participate in a closed 3 day consultation meeting, following formal comments submitted in January 2022 on global convergence among health authorities. The meeting objectives were to review a draft paper, discuss key issues identified from public consultation, reach consensus and propose improvements. The meeting was attended by ISCT representatives Karen Nichols, Esq. ISCT Chief Regulatory Officer and Gabrielle O’Sullivan PhD, Australia and New Zealand LRA Co-Chair.
Meeting with Representatives of the Pharmaceutical Industry on the Hospital Exemption (November 23, 2022)
ISCT was invited to participate in a closed meeting with relevant organizations and subject matter experts to discuss implications for the pharma industry of the existence of exemption pathways for the manufacture of ATMPs, views of the pharma industry regarding the different implementation framework of Hospital Exemption in various member states in the EU. The meeting was attended by ISCT representatives Miguel Forte, MD, PhD ISCT President-Elect and Christopher Bravery, PhD, ISCT EU LRA Chair

Queenie Jang, BSc (Pharmacy), MBA
Chief Executive Officer
Canada
ISCT has made significant headway in offering leading educational materials for stakeholders, through renewed partnerships across the globe. From translational research to good manufacturing practices and regulatory understanding, ISCT members now have more access than ever to the key knowledge and networks that they need to become an effective part of the fast-expanding CGT workforce.
ISCT has focused its investment in education towards manufacturing this year, to address a critical demand for expertise in the global market. By expanding pathways for professionals to become key players in the sector, ISCT is opening doors for newcomers to the field.
Our Society continues to make major impacts. Throughout 2021, we have made strategic investments in several key resources, including our redesigned website and member library, and the development of educational programs targeting areas of critical importance in the CGT workforce. More than ever, our focus is on cultivating the emerging generation of cell and gene therapy professionals, promoting high standards at a global level, and providing the platform for cutting-edge knowledge and practices to flow through the sector.
Alongside this, our community continues to show its vibrant spirit. In the past year, we have seen the first iteration of a highly successful ISCT TV program. We’ve also seen the emergence of a truly community-driven member forum, and thought-provoking discussions amongst our global membership. Underlying all of this was a highly successful ISCT 2021 New Orleans VIRTUAL Annual Meeting. Each of these initiatives plays a crucial role in keeping connections in the field ready and fresh despite the great challenges of the ongoing pandemic.
ISCT 2021 met with great success, despite the screen fatigue that has characterized much of the last year and a half as we continue to face the effects of the COVID-19 pandemic. Under the guidance of a strong organizing committee, we delivered a dynamic and LIVE-focused program to our global membership. The virtual program for this meeting has become a permanent resource for ISCT members across the sector.
Despite the difficulties of having to connect virtually, ISCT members and leaders have truly brought the globe together in advancing cell and gene therapies in this past year.
From the work of past ISCT president Massimo Dominici in kickstarting a strategic partnership with the WHO to develop the first harmonized CGT nomenclature, to work of our MSC committee in solidifying terminology, our Society is having a breakthrough year in the regulatory space.
We continue to collaborate with key sector partners like ICCBBA, while contributing guidances and contributions to regulatory agencies in the EU and Australia especially. Our collaborations with the FDA, meanwhile, led to a landmark webinar discussing the implications of the end of the Enforcement Discretion Period, a key discussion that has great value for stakeholders across the sector globally.
Alongside all of this, ISCT is working hard to address an urgent global need: the rising demand for cell and gene therapy professionals. Working with our strategic partners, we have created programs to help open doors for newcomers to the field. Beginning with the Andalusian Network for the Development and Translation of Advanced Therapies (ANd&tAT), we have created a flexible, digital program aimed at teaching best practices and principles for those working in cell manufacturing, especially in Europe. With our partners at CMaT, we are now developing a key program that aims to bridge a critical gap in the number of skilled personnel across companies and clinical manufacturing sectors in particular.
Workforce development is the greatest challenge that lies ahead for cell and gene therapies to come to full fruition. Our field is driven by the passion of a diverse and relatively small group of pioneers in regulatory, commercial, and research enterprises. To advance further, we must harness this passion to develop paths and open doors for both interested professionals and fresh newcomers alike. If this year has proven anything, it’s that ISCT members are ready to take on this critical challenge.
This year we saw, time and time again, ISCT members coming together to collaborate and advance our field
As a truly global organization, ISCT leverages a regional model to deploy solutions to global issues on a regional level. Spearheaded by passionate volunteer leadership, who understand the needs and concerns unique to each region, ISCT addresses the emergent needs of CGT professionals across the globe. With partnerships including regional partner organizations, regulatory bodies, and stakeholder communities, each of the ISCT regional committees drives the advancement of translational science at a high global standard.
Our committees work autonomously to deliver key scientific meetings, stimulate membership growth, and develop regional professional networks. Through 2022, our committees worked hard to develop the groundwork for new committees with a special focus on early-stage professionals and facilitated several major events that have continued to build consensus across the sector.
In 2022, to further the society’s commitment to workforce development, ISCT formed three new regional early-stage professionals subcommittees in Asia, Australia and New Zealand and South-Central America. Supported by the respective regional executive committee, these subcommittees were created to promote regional and global engagement and networking for early-stage professionals providing them with resources and career guidance to ensure their success.
Championed by regional volunteers, the Asia, Australia and New Zealand and South-Central America subcommittees are in full swing developing key initiatives to drive ESP membership and engagement in each region. ISCT is currently working on establishing ESP subcommittees in both North America and Europe for 2023.
ISCT regional committees deliver high-quality programmes globally, with autonomous planning supported by the society’s global network and resources. As restrictions eased post-pandemic, regional executive committees were excited to return to in-person meetings and reconnect face-to-face with the wider ISCT community.
With a reignited vigour to connect cell and gene therapy professionals globally, 2022 was a particularly active year for regional events. ISCT regional committees partnered and collaborated with several external groups and organizations as well connecting with other ISCT committees.
In August, the Australia & New Zealand Regional Executive Committee hosted its first in-person regional meeting post-pandemic. The highly anticipated event welcomed 137 delegates to Brisbane, Australia, and featured workshops and panel discussions with particular attention to quality, regulatory affairs, education, training, commercialization and clinical practice.
Meanwhile, in North America, the leadership team hosted a virtual interactive member-focused regional town hall meeting in October to connect and engage with members in the region. The meeting facilitated open discussions focused on workforce development and retention challenges, including both commercial and academic perspectives.
To facilitate cross-regional collaboration, The North America and South & Central America Regional Executive Committees partnered to present a two-part webinar series. The first installment discussed the clinical manufacturing of CAR T Cells, and the second explored the mechanism, safety and efficacy of mRNA-based cancer vaccines for oncological therapy. The collaborative series brought together subject matter experts and key opinion leaders from across both regions and received over 550 registrants from across the globe.


In Australia & New Zealand, ISCT leaders held a joint virtual scientific meeting with the Biotherapeutics Association of Australasia (BAA) to address developments in cell and gene therapies. The two-day event, held in February 2022, hosted scientific presentations featuring both international and regional speakers to discuss the latest developments in the clinical and commercial delivery of cell and gene therapies and the latest regulatory updates relevant to the region.
Leaders in Asia were delighted to continue its partnership with the Korean Society of Blood and Marrow Transplantation (KSBMT) with a joint session on gene editing and gene therapy at the International Congress of Blood and Marrow Transplantation (ICBMT), the 27th Annual congress of KSBMT in September. The long-standing partnership between ISCT and KSBMT is a key partnership for the region. It fosters international cooperation in education and research in the field of hematopoietic stem cell transplantation and cell & gene therapy.


In May, the ISCT Asia Regional Executive Committee partnered with the Japanese Society for Transplantation and Cellular Therapy (JSTCT 2022) to host a joint session on ‘Strategies to Improve Classical CBT by Introducing New Cellular Therapies’. The session was chaired by Satoshi Takahashi, MD, PhD, Past ISCT Asia Regional Vice-President, JSTCT 2022 President and included a remote presentation from William YK Hwang, MBBS, MMed, FRCP, FAMS, ISCT Asia Regional Vice-President Elect.
In October, the South and Central America Executive Committee again partnered with the Brazilian Association of Cellular and Gene Therapy (ABTCel-Gen) for the XII Congress of ABTCel – Gen in Rio de Janeiro. ISCT invited speakers participated both in-person and virtually, leading discussions on topics such as manuscript writing, cell therapy and therapies for cancer through sessions and interactive workshops. Alongside event participation, The South and Central America regional leaders were proud to sponsor the top-scoring abstract awards and provide a special abstract supplement showcasing all accepted abstracts from the meeting publication in Cytotherapy, the official journal of ISCT.




Janet Macpherson, PhD
Australia

C. Russell Y. Cruz, MD, PhD
USA
Available to ISCT members, Telegraft is the go-to resource to keep up to date with the latest Society activities and important developments in cell and gene therapy.
2022 brought exciting updates for Telegraft, with the launch of the ‘Telegraft Hub,’ the new online home for ISCT news and community. Alongside monthly e-newsletters, ISCT members now have access to the online interactive hub, allowing them to connect with peers and access key updates and curated content.
The Telegraft Editorial Board works to engage and connect the whole ISCT community across academia, science, regulation and commercial by sharing insightful discussions and up-to-date information on critical issues affecting the field of cellular therapy.

Michele Sugrue, MT
USA

Wouter Van’t Hof, PhD
USA

Joaquim Vives, PhD
Portugal

Rounak Dubey, MBBS, MD
India

Shyam Bhakta, MD, MBA
India

Ashley Krull, BSc, PhD
USA

Cytotherapy®, the official journal for the International Society for Cell and Gene Therapy (ISCT), publishes novel and innovative results from high quality scientific and clinical studies in the fields of cell and gene therapy. In 2021, Cytotherapy has continued to successfully advance and expand the field of cell and gene therapy for clinical researchers, oncologists, hematologists, physicians, and regulatory experts alike. The Journal had a 6.196 impact factor for 2022, a new record from which the editorial board hopes to grow further.
You can read issues of Cytotherapy® here.
Follow Cytotherapy® on Twitter to stay up to date on all the latest news and publications.
In January 2022, Dr. Rachel Burga was appointed as the new assistant editor for Cytotherapy. With a background in biomedical engineering and immunology, Rachel is the Principal Scientist of Cell Therapy at Obsidian Therapeutics. She has been an active member of ISCT since 2016 and currently sits on the Early-Stage Professionals Committee as Co-Chair.
As Assistant Editor, Rachel will work closely with Senior Editor Dr. Donald G. Phinney and Commissioning Editor, Dr. Patrick Hanley, to raise awareness of the Cytotherapy brand. In her first term, Rached helped recruit outstanding content for the journal and work closely with the ISCT membership to ensure that the journal continues to support the Society’s mission.

Rachel Burga, PhD
Assistant Editor, Cytotherapy
United States
Each year, ISCT hosts the Insta-Your-Cells Photo Challenge, calling on community members to submit interesting images from under the microscope. These images are selected to feature on the front cover of Cytotherapy®, the Official Journal of ISCT, showcasing the fascinating world of cells to peers around the globe.
The winning image of the 3rd annual Insta Your Cells Photo Challenge was submitted by Philippe Cohen, MD, PhD, France. ISCT Head Office prepared a framed keepsake to celebrate this iconic first-place image.
Take a look at the stunning submissions selected to be featured on the front cover of Cytotherapy® in 2022.


Emily Hopewell, PhD
Indiana University School Of Medicine
United States

Patrick Hanley, PhD
Children’s National Hospital
United States
Recognizing the current skills gaps in CGT translation as a critical issue for the sector, in November 2022, ISCT formed the ISCT Workforce Development Committee.
Led by Co-chairs Emily Hopewell, PhD and Patrick Hanley, PhD the committee will provide strategic guidance to identify, develop and deliver ISCT training and development programs.
Programs will focus on the specific needs of professions based on their region, industry, and skill level to support the sustainable growth of the cell and gene therapy sector.
Throughout 2023, the committee will focus on identifying and prioritizing urgent needs for training and educational programs for professionals working across the full spectrum of translational science, starting from pre-clinical development through to patient access, to develop and deliver comprehensive programs to upskill and train the existing CGT workforce.


Laertis Ikonomou, PhD
The State University of New York at Buffalo
United States
In October 2021, the ISCT Presidential Task Force on the Use of Unproven and/or Unethical Cell and Gene Therapies formally became the ISCT Committee on the Ethics of Cell and Gene Therapy (ECGT). This change is intended to reflect the committees revised mission to identify key ethical issues associated with the development, regulatory authorization, and distribution of cell and gene therapies.
In 2022, the Committee re-affirmed its position on, and concerns with, speculative immune cell banking. Following the 2022 US federal court ruling in favor of California Stem Cell Treatment Center, Inc., and Cell Surgical Network Corporation, the Committee contributed to publishing an ISCT position and press release, highlighting the dangers of unproven cell therapies to patient safety.
Undertaking its broader remit, the ECGT Committee developed and published informed positions providing guidance and clarity on complicated ethical matters within the CGT space. The Committee has undertaken the task of developing resources to address ethical topics, and potential issues, arising from the EU Hospital Exemption and the US expanded access pathway. The Committee organized a roundtable session at the ISCT 2022 San Francisco Annual Meeting addressing both pathways, and published a position paper on Hospital Exemption within the framework of safety and ethical interests of patients. On January 19, 2022, the Committee launched the ISCT Expanded Access Working Group.
Over the course of 2022, the Committee has worked to develop a guide for the general public, which analyses the regenerative medicine industry, focusing on distinctive features of unproven cell and cell-based products, suggesting practical strategies to address marketing of such products, and providing an overview of reporting mechanisms available to patients across different jurisdictions globally. This comprehensive guide will be published in 2023 and provides an important resource to protect patients globally.

Patricia J. Zettler, JD
The Ohio State University
United States

The ISCT Expanded Access Working Group was launched with the mandate to identify and address practical, ethical, and regulatory issues that arise from the use, and potential misuse, of the expanded access pathway by various CGT stakeholders.
Since its launch, the Working Group has been developing informed perspectives on expanded access to generate educational resources that promote innovation in translational research while upholding the highest ethical standards. The Working Group submitted a short report reviewing the history and current state of the US expanded access pathway, and highlighting three emerging areas of concern. The report will be published in Cytotherapy in early 2023.
The overarching objective of the Working Group is to further refine its position on ethical issues arising from the use, and potential misuse, of the expanded access pathway, culminating in the development of a code of ethics. In developing this code of ethics, ISCT will seek out input and endorsement from other organizations in the field.
ISCT acknowledges the service of its members, volunteering expertise, time, and energy to the vast task of advancing the field of cell and gene therapies.
From mentoring the next generation to publishing key standards and fostering relationships across the sector, ISCT members set the gold standard.
We acknowledge these Society leaders who have completed terms of service in a formal position in 2021.
Your hard work and dedication have continually pushed the society to new heights, and we cannot wait to connect again soon:

John Rasko, AO, MBBS, PhD, FRCPA, FRACP, FAHMS
Chair, Strategic Advisory Council (Immediate Past President)
June 2020 – June 2022
Royal Prince Alfred Hospital
Sydney, Australia

Lizette Caballero, BS, MT(ASCP)
Global Secretary
June 2019 – June 2022
UCSF Blood and Marrow Transplant Lab
San Francisco, CA, United States

Rajiv Khanna, PhD AO
Australia & New Zealand, Regional Vice-President
June 2020 – June 2022
QIMR Berghofer Medical Research Institute
Brisbane, Australia

Mitchell Cairo, MD
North America, Regional Vice-President
June 2020 – June 2022
New York Medical College
New York, NY, United States

Karen Nichols, Esq.
Chief Regulatory Officer
March 2016 – June 2022
Vertex Pharmaceuticals
Cambridge, MA, United States

Vicki Antonenas, BSc, MSc
Elected Member Technologist
June 2020 – June 2022
Sydney Cellular Therapies Laboratory,
Westmead Hospital
Australia

Patrick Hanley, PhD
Co-Chair Immuno-Gene
Therapy Committee
Children’s National Hospital
United States

Giuseppe Orlando, MD, PhD
Co-Chair Gastrointestinal Committee
Wake Forest University
United States

Rachele Ciccocioppo, MD
Co-Chair Gastrointestinal Committee
University of Verona
Italy

George Muschler, MD
Co-Chair Orthopedic & Musculoskeletal Therapies
Cleveland Clinic
United States

Christian Jorgensen, MD, PhD
Co-Chair Orthopedic & Musculoskeletal Therapies
University Hospital Lapeyronie
France

Sarah Chan, Ma PhD
ECGT Committee
University of Edinburgh
United Kingdom

Andrew Hoffman, DVM, DVSc
Exosomes Committee
University of Pennsylvania
United States

John Fink, MBA
Co-Chair Process Development and Manufacturing Committee
PerkinElmer, Inc.
United States

Emily Hopewell, PhD
Indiana University School of Medicine
United States

Aaron Tze Kai Tan
Stanford University
United States

Lizbeth Diaz Polo, MD
University of San Martin de Porres
Spain

Jordan Greenberg, PhD
Shoreline Biosciences
United States

Shabnum Patel, PhD
CARGO Therapeutics
United States

Nancy H. Collins, PhD
University of Toledo
Toledo, OH, United States

Satyam Arora, MD
Super Speciality Paediatric Hospital and Post Graduate Teaching Institute
India

Lab Practices Committee
Vicki Antonenas, BSc, MSc
Westmead Hospital
Australia

Europe Legal & Regulatory Affairs Committee
Paula Salmikangas, PhD
NDA Group
Finland

Cytotherapy®, the official journal for the International Society for Cell and Gene Therapy (ISCT), publishes novel and innovative results from high quality scientific and clinical studies in the fields of cell and gene therapy.In 2021, Cytotherapy has continued to successfully advance and expand the field of cell and gene therapy for clinical researchers, oncologists, hematologists, physicians, and regulatory experts alike. The Journal had a 5.414 impact factor for 2020, a new record from which the editorial board hopes to grow further.
This year, ABTCel-Gen, a CGT-focused society based in South & Central America, joined as an affiliate society to Cytotherapy. This landmark partnership creates a strong pathway to publication for affiliate members, while drawing from a proven pool of translational expertise.
Take a look at our Talking with Giants series, launched in 2019, which showcases leaders in the field of cell and gene therapy through informal question and answer articles. Talking with Giants is featured at the start of each issue of Cytotherapy.
You can read issues of Cytotherapy® here.
This year, Cytotherapy® also launched a Twitter account to share fresh updates and resources.
Each year, ISCT hosts the Insta-Your-Cells Photo Challenge, calling on community members to submit interesting images from under the microscope. These images are selected to feature on the front cover of Cytotherapy®, the Official Journal of ISCT, showcasing the fascinating world of cells to peers around the globe.
This year, ISCT Head Office also prepared a framed keepsake to celebrate an iconic first place image, submitted by Pradyut Paul, PhD, University of Wisconsin-Madison, United States.

Take a look at the stunning submissions selected to be featured on the front cover of Cytotherapy® this year.



Janet Macpherson, PhD
Australia

C. Russell Y. Cruz, MD, PhD
USA
Available to ISCT members, Telegraft is the go-to resource to keep up to date with the latest Society activities and important developments in cell and gene therapy.
2022 brought exciting updates for Telegraft, with the launch of the ‘Telegraft Hub,’ the new online home for ISCT news and community. Alongside monthly e-newsletters, ISCT members now have access to the online interactive hub, allowing them to connect with peers and access key updates and curated content.
The Telegraft Editorial Board works to engage and connect the whole ISCT community across academia, science, regulation and commercial by sharing insightful discussions and up-to-date information on critical issues affecting the field of cellular therapy.

Nancy H. Collins, PhD
USA

Satyam Arora, MD
USA

Rounak Dubey, MBBS, MD
India

Shyam Bhakta, MD, MBA
India

Ashley Krull, BSc, PhD
USA

Michele Sugrue, MT
USA

Wouter Van’t Hof, PhD
USA

Joaquim Vives, PhD
Portugal
ISCT acknowledges the service of its members, volunteering expertise, time, and energy to the vast task of advancing the field of cell and gene therapies.
From mentoring the next generation to publishing key standards and fostering relationships across the sector, ISCT members set the gold standard.
We acknowledge these Society leaders who have completed terms of service in a formal position in 2021.
Your hard work and dedication have continually pushed the society to new heights, and we cannot wait to connect again soon:

John Rasko, AO, MBBS, PhD, FRCPA, FRACP, FAHMS
Chair, Strategic Advisory Council (Immediate Past President)
June 2020 – June 2022
Royal Prince Alfred Hospital
Sydney, Australia

Lizette Caballero, BS, MT(ASCP)
Global Secretary
June 2019 – June 2022
UCSF Blood and Marrow Transplant Lab
San Francisco, CA, United States

Rajiv Khanna, PhD AO
Australia & New Zealand, Regional Vice-President
June 2020 – June 2022
QIMR Berghofer Medical Research Institute
Brisbane, Australia

Mitchell Cairo, MD
North America, Regional Vice-President
June 2020 – June 2022
New York Medical College
New York, NY, United States

Karen Nichols, Esq.
Chief Regulatory Officer
March 2016 – June 2022
Vertex Pharmaceuticals
Cambridge, MA, United States

Vicki Antonenas, BSc, MSc
Elected Member Technologist
June 2020 – June 2022
Sydney Cellular Therapies Laboratory,
Westmead Hospital
Australia

Patrick Hanley, PhD
Co-Chair Immuno-Gene
Therapy Committee
Children’s National Hospital
United States

Giuseppe Orlando, MD, PhD
Co-Chair Gastrointestinal Committee
Wake Forest University
United States

Rachele Ciccocioppo, MD
Co-Chair Gastrointestinal Committee
University of Verona
Italy

George Muschler, MD
Co-Chair Orthopedic & Musculoskeletal Therapies
Cleveland Clinic
United States

Christian Jorgensen, MD, PhD
Co-Chair Orthopedic & Musculoskeletal Therapies
University Hospital Lapeyronie
France

Sarah Chan, Ma PhD
ECGT Committee
University of Edinburgh
United Kingdom

Andrew Hoffman, DVM, DVSc
Exosomes Committee
University of Pennsylvania
United States

John Fink, MBA
Co-Chair Process Development and Manufacturing Committee
PerkinElmer, Inc.
United States

Emily Hopewell, PhD
Indiana University School of Medicine
United States

Aaron Tze Kai Tan
Stanford University
United States

Lizbeth Diaz Polo, MD
University of San Martin de Porres
Spain

Jordan Greenberg, PhD
Shoreline Biosciences
United States

Shabnum Patel, PhD
CARGO Therapeutics
United States

Nancy H. Collins, PhD
University of Toledo
Toledo, OH, United States

Satyam Arora, MD
Super Speciality Paediatric Hospital and Post Graduate Teaching Institute
India

Lab Practices Committee
Vicki Antonenas, BSc, MSc
Westmead Hospital
Australia

Europe Legal & Regulatory Affairs Committee
Paula Salmikangas, PhD
NDA Group
Finland
As a truly global organization, ISCT leverages a regional model to deploy solutions to global issues on a regional level. Spearheaded by passionate volunteer leadership, who understand the needs and concerns unique to each region, ISCT addresses the emergent needs of CGT professionals across the globe. With partnerships including regional partner organizations, regulatory bodies, and stakeholder communities, each of the ISCT regional committees drives the advancement of translational science at a high global standard.
Our committees work autonomously to deliver key scientific meetings, stimulate membership growth, and develop regional professional networks. Through 2022, our committees worked hard to develop the groundwork for new committees with a special focus on early-stage professionals and facilitated several major events that have continued to build consensus across the sector.
In 2022, to further the society’s commitment to workforce development, ISCT formed three new regional early-stage professionals subcommittees in Asia, Australia and New Zealand and South-Central America. Supported by the respective regional executive committee, these subcommittees were created to promote regional and global engagement and networking for early-stage professionals providing them with resources and career guidance to ensure their success.
Championed by regional volunteers, the Asia, Australia and New Zealand and South-Central America subcommittees are in full swing developing key initiatives to drive ESP membership and engagement in each region. ISCT is currently working on establishing ESP subcommittees in both North America and Europe for 2023.
ISCT regional committees deliver high-quality programmes globally, with autonomous planning supported by the society’s global network and resources. . As restrictions eased post-pandemic, regional executive committees were excited to return to in-person meetings and reconnect face-to-face with the wider ISCT community.
With a reignited vigour to connect cell and gene therapy professionals globally, 2022 was a particularly active year for regional events. ISCT regional committees partnered and collaborated with several external groups and organizations as well connecting with other ISCT committees.
In August, the Australia & New Zealand Regional Executive Committee hosted its first in-person regional meeting post-pandemic. The highly anticipated event welcomed 137 delegates to Brisbane, Australia, and featured workshops and panel discussions with particular attention to quality, regulatory affairs, education, training, commercialization and clinical practice.
Meanwhile, in North America, the leadership team hosted a virtual interactive member-focused regional town hall meeting in October to connect and engage with members in the region. The meeting facilitated open discussions focused on workforce development and retention challenges, including both commercial and academic perspectives.
To facilitate cross-regional collaboration, The North America and South & Central America Regional Executive Committees partnered to present a two-part webinar series. The first installment discussed the clinical manufacturing of CAR T Cells, and the second explored the mechanism, safety and efficacy of mRNA-based cancer vaccines for oncological therapy. The collaborative series brought together subject matter experts and key opinion leaders from across both regions and received over 550 registrants from across the globe.


In Australia & New Zealand, ISCT leaders held a joint virtual scientific meeting with the Biotherapeutics Association of Australasia (BAA) to address developments in cell and gene therapies. The two-day event, held in February 2022, hosted scientific presentations featuring both international and regional speakers to discuss the latest developments in the clinical and commercial delivery of cell and gene therapies and the latest regulatory updates relevant to the region.
Leaders in Asia were delighted to continue its partnership with the Korean Society of Blood and Marrow Transplantation (KSBMT) with a joint session on gene editing and gene therapy at the International Congress of Blood and Marrow Transplantation (ICBMT), the 27th Annual congress of KSBMT in September. The long-standing partnership between ISCT and KSBMT is a key partnership for the region. It fosters international cooperation in education and research in the field of hematopoietic stem cell transplantation and cell & gene therapy.


In May, the ISCT Asia Regional Executive Committee partnered with the Japanese Society for Transplantation and Cellular Therapy (JSTCT 2022) to host a joint session on ‘Strategies to Improve Classical CBT by Introducing New Cellular Therapies’. The session was chaired by Satoshi Takahashi, MD, PhD, Past ISCT Asia Regional Vice-President, JSTCT 2022 President and included a remote presentation from William YK Hwang, MBBS, MMed, FRCP, FAMS, ISCT Asia Regional Vice-President Elect.
In October, the South and Central America Executive Committee again partnered with the Brazilian Association of Cellular and Gene Therapy (ABTCel-Gen) for the XII Congress of ABTCel – Gen in Rio de Janeiro. ISCT invited speakers participated both in-person and virtually, leading discussions on topics such as manuscript writing, cell therapy and therapies for cancer through sessions and interactive workshops. Alongside event participation, The South and Central America regional leaders were proud to sponsor the top-scoring abstract awards and provide a special abstract supplement showcasing all accepted abstracts from the meeting publication in Cytotherapy, the official journal of ISCT.

Building Consensus and Community
Motivated to stay true to the essence of ISCT as a truly global translation-focused community of experts, ISCT made major strategic progress in 2022, with significant investments in community development, workforce development and addressing ethical issues. Working in collaboration with ISCT leadership, the Society enhanced the value of its membership value across all disciplines for leaders and newcomers alike as well as developed key resources and drove consensus to combat the critical issues facing the wider CGT sector. As the ISCT community continues to grow, the Society is perfectly positioned to empower the field to drive the translation of research into safe, effective and accessible treatments with long-lasting impact.

Excited to welcome delegates to the first in-person ISCT meeting post Pandemic, ISCT was determined to make a significant impact with ISCT 2022 San Francisco. Welcoming 1,646 delegates from across the globe, ISCT 2022 was the largest ISCT Annual Meeting to date and was proud to serve as a key event reconnecting the wider CGT community. A standout feature of the meeting was the redesign of the ISCT Scientific program, a strategic initiative inspired to better cater to the interests of all delegates, across the full range of CGT development and deployment.
The Translational Pathway Program, reflects ISCT’s role in driving the translation of scientific research into safe and effective therapies that can be deployed to patients. The new program was designed to mirror the therapeutic development process and integrated perspectives across the CGT sector to address key topics at all stages of translation.
As a counter-part to the Translational Pathway Program, ISCT also launched the Roundtable Program at ISCT 2022. Unique in the cell and gene therapy sector, this program provides a platform for delegates to debate and collaboratively develop consensus and solutions to practical problems and barriers in the field. Recognizing the breadth and depth in expertise of Annual Meeting delegates, ISCT developed this unique platform to connect professionals from across the globe who share the same goals and encounter the same difficulties.
“ISCT broadened its focus, through a whole series of roundtables, to cover wider challenges experienced across the industry, from generating return on investment to managing the supply chain. ISCT will continue to monitor the efficacy of solutions generated at the meeting and continue to work with all stakeholders to ensure an increasing number of patients are able to benefit from cell and gene therapies.”

Bruce Levine, PhD
University Of Pennsylvania
United States
The rapid growth of the sector has introduced new ethical concerns associated with the development, regulatory authorization, and distribution of cell and gene therapies. Recognizing this, ISCT has worked to develop internal infrastructure so that the Society remains a leading voice on critical and topical ethical issues.
Undertaking a broader remit to identify these key ethical issues, ISCT has introduced the Committee on the Ethics of Cell and Gene Therapy (ECGT). Formally the ISCT Presidential Task Force on the Use of Unproven and/or Unethical Cell and Gene Therapies, this new multidisciplinary committee regroups key stakeholder perspectives to promote the ethical development of the sector.
To further refine the Society’s position on ethical issues, ISCT formed the ISCT Expanded Access Working Group. The overarching objective of the Working Group is to further refine its position on ethical issues arising from the use, and potential misuse, of the expanded access pathway, culminating in the development of a code of ethics.
Under the guidance of the new ECGT committee, ISCT is now better positioned to give informed consensus on ethical issues. In 2022, following the 2022 US federal court ruling in favor of California Stem Cell Treatment Center, Inc., and Cell Surgical Network Corporation, ISCT published a press release highlighting the dangers of unproven cell therapies to patient safety.
With Industry members representing a vital part of ISCT DNA, in 2022, ISCT worked to adapt the industry program to better meet the needs of our growing community. In collaboration with the ISCT Industry leadership, ISCT worked to evaluate how to adapt our program gaining feedback from our members at the forefront of driving the translation of cell and gene therapies.
At its core, ISCT Industry Membership provides the ability to network, discuss current challenges in Cell and Gene Therapy and examine potential solutions with global experts from a variety of disciplines. As part of the restructure, ISCT has adapted the membership program to two levels, Patron and Associate, both of which offer access to networking events, monthly strategic discussion forums and the opportunity to contribute to the development of sector advancing educational resources.
The newly enhanced program also welcomed The Asia Pacific Regional Commercialization Committee, created to foster strong industry relationships in the region.
The launch of the new membership program marks a significant milestone for ISCT, with a record number of new members joining in 2022. ISCT Industry leadership looks forward to continuing to grow our global community and support those working to expand the development and delivery of safe and effective cell and gene therapies for patients.

Anthony Ting, PhD
Bone Therapeutics
United States
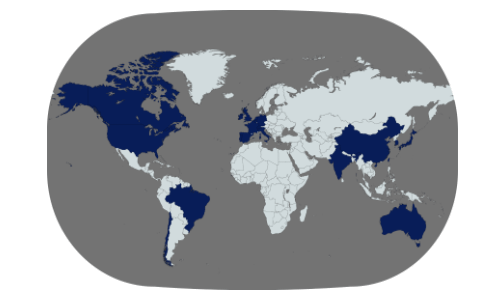
ISCT continues to advance scientific translation through the expansion and improvement of its peer-reviewed scientific journal, Cytotherapy. Under the close collaboration of its editorial board, with support from ISCT Head Office and our publishers at Elsevier, the Journal has attained a record-breaking impact factor at 6.196.
In 2022, Cytotherapy published 132 articles including, reviews, short repots, correspondence and editorials. The Journal received a record number of downloads and citations solidifying its position as the first choice in cell and gene therapy translation publishing.

Donald Phinney, PhD
Senior Editor
United States

Patrick Hanley, PhD
Commissioning Editor
United States
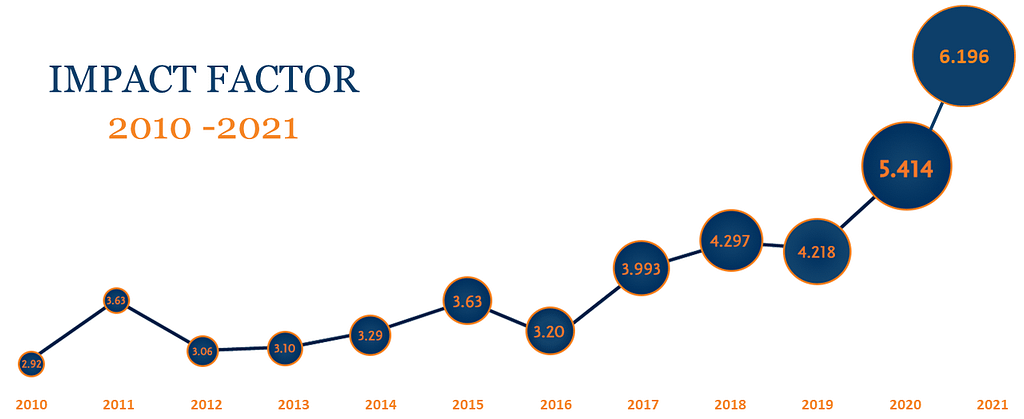
Take a look at the stunning submissions selected to be featured on the front cover of Cytotherapy® in 2022.
Building Consensus and Community
Motivated to stay true to the essence of ISCT as a truly global translation-focused community of experts, ISCT made major strategic progress in 2022, with significant investments in community development, workforce development and addressing ethical issues. Working in collaboration with ISCT leadership, the Society enhanced the value of its membership value across all disciplines for leaders and newcomers alike as well as developed key resources and drove consensus to combat the critical issues facing the wider CGT sector. As the ISCT community continues to grow, the Society is perfectly positioned to empower the field to drive the translation of research into safe, effective and accessible treatments with long-lasting impact.

Excited to welcome delegates to the first in-person ISCT meeting post Pandemic, ISCT was determined to make a significant impact with ISCT 2022 San Francisco. Welcoming 1,646 delegates from across the globe, ISCT 2022 was the largest ISCT Annual Meeting to date and was proud to serve as a key event reconnecting the wider CGT community. A standout feature of the meeting was the redesign of the ISCT Scientific program, a strategic initiative inspired to better cater to the interests of all delegates, across the full range of CGT development and deployment.
The Translational Pathway Program, reflects ISCT’s role in driving the translation of scientific research into safe and effective therapies that can be deployed to patients. The new program was designed to mirror the therapeutic development process and integrated perspectives across the CGT sector to address key topics at all stages of translation.
As a counter-part to the Translational Pathway Program, ISCT also launched the Roundtable Program at ISCT 2022. Unique in the cell and gene therapy sector, this program provides a platform for delegates to debate and collaboratively develop consensus and solutions to practical problems and barriers in the field. Recognizing the breadth and depth in expertise of Annual Meeting delegates, ISCT developed this unique platform to connect professionals from across the globe who share the same goals and encounter the same difficulties.
“ISCT broadened its focus, through a whole series of roundtables, to cover wider challenges experienced across the industry, from generating return on investment to managing the supply chain. ISCT will continue to monitor the efficacy of solutions generated at the meeting and continue to work with all stakeholders to ensure an increasing number of patients are able to benefit from cell and gene therapies.”

Bruce Levine, PhD
University Of Pennsylvania
United States
The rapid growth of the sector has introduced new ethical concerns associated with the development, regulatory authorization, and distribution of cell and gene therapies. Recognizing this, ISCT has worked to develop internal infrastructure so that the Society remains a leading voice on critical and topical ethical issues.
Undertaking a broader remit to identify these key ethical issues, ISCT has introduced the Committee on the Ethics of Cell and Gene Therapy (ECGT). Formally the ISCT Presidential Task Force on the Use of Unproven and/or Unethical Cell and Gene Therapies, this new multidisciplinary committee regroups key stakeholder perspectives to promote the ethical development of the sector.
To further refine the Society’s position on ethical issues, ISCT formed the ISCT Expanded Access Working Group. The overarching objective of the Working Group is to further refine its position on ethical issues arising from the use, and potential misuse, of the expanded access pathway, culminating in the development of a code of ethics.
Under the guidance of the new ECGT committee, ISCT is now better positioned to give informed consensus on ethical issues. In 2022, following the 2022 US federal court ruling in favor of California Stem Cell Treatment Center, Inc., and Cell Surgical Network Corporation, ISCT published a press release highlighting the dangers of unproven cell therapies to patient safety.
With Industry members representing a vital part of ISCT DNA, in 2022, ISCT worked to adapt the industry membership program to better meet the needs of our growing community. In collaboration with ISCT Industry leadership, ISCT worked to tailor its Committees using feedback from our members to create a program that supports those who are at the forefront of driving the translation of cell and gene therapies.
At its core, ISCT Industry Membership provides the ability to network, discuss current challenges in Cell and Gene Therapy and examine potential solutions with global experts from a variety of disciplines. As part of the restructure, ISCT has adapted the membership program to two levels, Patron and Associate, both of which offer access to monthly strategic discussion forums, networking events, and the opportunity to contribute to the development of sector advancing educational resources.
The newly enhanced program also welcomed The Asia Pacific Regional Commercialization Committee, created to foster strong industry relationships in the region.
The launch of the new membership program marks a significant milestone for ISCT, with a record number of new members joining in 2022. ISCT Industry leadership looks forward to continuing to grow the ISCT global industry community and support those working to expand the development and delivery of safe and effective cell and gene therapies for patients.

Anthony Ting, PhD
BRL C> Consulting
United States

ISCT continues to advance scientific translation through the expansion and improvement of its peer-reviewed scientific journal, Cytotherapy. Under the close collaboration of its editorial board, with support from ISCT Head Office and our publishers at Elsevier, the Journal has attained a record-breaking impact factor at 6.196.
In 2022, Cytotherapy published 132 articles including, reviews, short repots, correspondence and editorials. The Journal received a record number of downloads and citations solidifying its position as the first choice in cell and gene therapy translation publishing.

Donald Phinney, PhD
Senior Editor
United States

Patrick Hanley, PhD
Commissioning Editor
United States

Take a look at the stunning submissions selected to be featured on the front cover of Cytotherapy® in 2022.

Bruce Levine, PhD
President
June 2020 – June 2022
Barbara and Edward Netter Professor in Cancer Gene Therapy
University of Pennsylvania
Philadelphia, PA, United States

Jacques Galipeau, MD
President-Elect
June 2020 – June 2022
University of Wisconsin-Madison
Madison, WI, United States

John Rasko, AO, MBBS, PhD, FRCPA, FRACP, FAHMS
Chair, Strategic Advisory Council (Immediate Past President)
June 2020 – June 2022
Royal Prince Alfred Hospital
Sydney, Australia

Lizette Caballero, BS, MT(ASCP)
Global Secretary
June 2019 – June 2022
UCSF Blood and Marrow Transplant Lab
San Francisco, CA, United States

Emily Hopewell, PhD
Global Treasurer
June 2020 – June 2023
Indiana University School of Medicine
Zionsville, Indiana

Bryan Choi, PhD
Asia, Regional Vice-President
June 2021 – June 2023
Strategic Center For Regenerative Medicine (SCRM)
Inha University
Incheon, Korea


Massimiliano Gnecchi, MD, PhD
Europe, Regional Vice-President
June 2021 – June 2023
University Of Pavia & IRCCS Policlinico San Matteo Hospital
Pavia, Italy

Mitchell Cairo, MD
North America, Regional Vice-President
June 2020 – June 2022
New York Medical College
New York, NY, United States

Antonio Carlos Campos de Carvalho, MD, PhDSouth and Central America, Regional Vice-President
June 2021 – June 2023
Institute Of Biophysics
Federal University Of Rio De Janeiro
Rio De Janeiro, Brazil

Daniel J. Weiss, MD, PhD
Chief Scientific Officer
June 2016 – June 2022
University of Vermont
Burlington, VT, United States

Anthony Ting, PhD
Chief Commercialization Officer
May 2020 – May 2022
Athersys
Cleveland, OH, United States

Karen Nichols, Esq.
Chief Regulatory Officer
March 2016 – June 2022
Vertex Pharmaceuticals
Cambridge, MA, United States

Dominique Farge, MD, PhD
Elected Member MD
June 2021 – June 2023
Greater Paris University Hospitals – AP-HP
Paris, France

Sowmya Viswanathan, PhD
Elected Member PhD
June 2020 – June 2022
University Health Network and University of Toronto
Canada

Vicki Antonenas, BSc, MSc
Elected Member Technologist
June 2020 – June 2022
Sydney Cellular Therapies Laboratory,
Westmead Hospital
Australia

Diane Kadidlo, BSc, MT(ASCP), SBB
Elected Member Technologist
June 2021 – June 2023
Molecular And Cellular Therapeutics
University Of Minnesota
St Paul, Minnesota, United States

Donald Phinney, PhD
Senior Editor of the Journal
October 2018 – Present
Professor at The Scripps Research Institute
Jupiter, FL, United States

Queenie Jang, BSc (Pharmacy), MBA
Chief Executive Officer
ISCT
Vancouver, BC, Canada



Kevin Bosse, PhD RAC
Nationwide
Children’s Hospital
United States
Rachel Burga, PhD
Obsidian Therapeutics
United States
The ISCT Early Stage Professionals Committee (ESP) strives to engage and connect ESP with the wider CGT sector on a regional and global level. Co-Chaired by Kevin Bosse, PhD RAC and Rachel Burga, PhD, the committee provides networking opportunities and career development resources for professionals to excel in their careers.
The committee hosts a variety of tailored events and workshops, such as the ISCT ESP Mentoring Program and the ISCT ESP Leadership Development program helping connect professionals with key opinion leaders and subject matter experts.
In 2022, ISCT formed three regional ESP subcommittees in Asia, Australia and New Zealand and South Central America to drive ESP membership and increase interactions between ESPs and regional leadership.