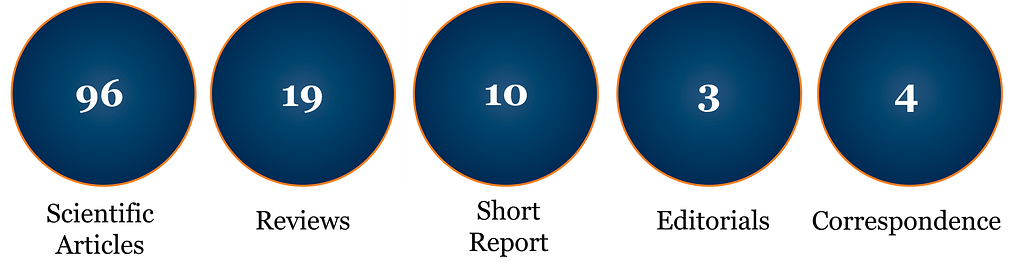
Anthony Ting, PhD
Chief Commercialization Officer, ISCT
USA
Bringing together a diverse membership to advance CGT
“To continue to be a leader, ISCT needs to identify the critical needs of our industry and use our unique membership of academia, industry and regulatory bodies to provide solutions”
We sat down with incoming Chief Commercialization Officer, Anthony Ting, to learn more about his vision for the ISCT Industry Community
“As the decade continues, we will see improvements in technologies (e.g. gene modification) and expansion of cell and gene therapy to multiple cell types and indications that have not previously been considered,” he says. “During my tenure as Chief Commercialization Officer, I hope to increase the exposure of the early-stage professionals (ESPs) to industry leaders, to expand our membership to include more members of large pharma, to drive technologies that will further enable cell and gene therapy, and most importantly, to consider the patient experience.”
The Chief Commercialization Officer (CCO) is a role within ISCT that provides strategic oversight on the positioning of the Society, as an effective and critical voice in the cell and gene therapy industry. The CCO ensures the Society addresses the needs of Industry members.
In a year in which cell and gene therapies have experienced record public interest and increased funding at a global level, we welcome Dr. Anthony Ting, an established translational leader with successful experience overseeing global product development, as CCO.
“The greatest challenge we face is the success of cell and gene therapy,” he observes. “There has been an expansion of other societies and the entrance of large pharma in the cell and gene space. To continue to be a leader, ISCT needs to identify the critical needs of our industry and use our unique membership of academia, industry and regulatory bodies to provide solutions.”
The acceleration of cell and gene therapies means that consensus on the critical issues facing stakeholders with a breadth of interests becomes increasingly important. There is an increased need for dialogue between the diverse member groups within the society, and the interests they represent to enable therapies to reach the market.
ISCT is poised to conduct this kind of dialogue, and Dr. Ting sees a proven model that can be expanded. “In a relaxed environment with ISCT industry members, we are allowed ample time and access to key players in the space and one can truly see how the camaraderie of ISCT enables the growth of our industry,” remarks Dr. Ting. “Some of my fondest memories of ISCT are the Industry Networking receptions that took place at the British Ambassador’s residence in France, and at the House of Parliament in London. I can speak personally to the value of this kind of global industry networking in the Society, and I can see it extending beyond, engaging all of our member groups.”

Rachele Ciccocioppo, MD
Deputy Chief Scientific Officer, ISCT
Italy
Cultivating a Community for Scientific Success
“All this work may be realized only within a strong, global Community, like ours at ISCT …So, I invite everybody interested to work with us to promote the cultural changes required for these emerging tools to find their rightful place within the therapeutic armamentarium”
“Undoubtedly, we are witnessing a tremendous expansion of the field of cell and gene therapies worldwide, and this represents an extraordinary opportunity for our patients,” says Rachele Ciccocioppo, ISCT Deputy Chief Scientific Officer, “As with all such opportunities, this brings positive and negative aspects that we must proactively manage.”
To address the increasing need to augment our scientific voice, ISCT has introduced the role of Deputy Chief Scientific Officer (DCSO). As the inaugural DCSO, Dr. Rachele Ciccocioppo is tasked with the oversight of ISCT scientific committees, building internal consensus, standards, and positions that continue to advance ISCT scientific leadership.
Dr. Ciccocioppo brings significant experience as both an established scientific leader, active across several medical and scientific societies; and, as a well-regarded medical researcher with recognized expertise in internal medicine and gastroenterology.
“In order to ensure ground-breaking scientific advances can be realized, it will be vital to cultivate a professional workforce, including bioengineers, health economists and bioinformatics. Any advancement in this area requires the collaboration of an expert network consisting of many kinds of scientists,” she says, identifying some of the key challenges facing the translation of CGT. To Dr. Ciccocioppo, as more cell and gene therapies begin to enter a translational space, an increased need for professionals with roots in science, and in the skills needed for manufacturing and market implementation, is becoming apparent.
She continues, “…Another challenge is the harmonization of the regulatory framework, and the possibility to offer this kind of therapies all around the world, while preventing exploitative gaps like medical tourism. The ability to effect harmonization of all these disparate but related disciplines is one of the exciting things about being part of ISCT.” As an experienced researcher herself, Dr. Ciccocioppo describes the importance of cooperation across regulators and researchers, pointing out that clear guidelines for market authorization and clinical trial research are a necessary early step that requires input from across a wide stakeholder community.
Drawing as well on her experience as a medical practitioner, Dr. Ciccocioppo concludes, “…Finally, a pillar of my mission will be to increase awareness of these therapies, which carry the advantage to perfectly fit within the needs of systems biology and precision medicine.” Dr. Ciccocioppo is a strong proponent of the use of cellular therapies for internal medicine, and has investigated the potential of Mesenchymal Stromal Cells as a treatment option for coeliac and inflammatory bowel diseases, as well as autoimmune enteropathy.
When asked how these goals can be achieved, she replies with enthusiasm, “All this work may be realized only within a strong, global Community, like ours at ISCT, where I have enjoyed the privilege of sharing ideas and projects. So, I invite everybody interested to work with us to promote the cultural changes required for these emerging tools to find their rightful place within the therapeutic armamentarium, and progress to become reality!”

Jaap Jan Boelens, MD, PhD
Chair, Stem Cell Engineering Committee
Memorial Sloan Kettering Cancer Center
New York, NY, United States
“Developments in this space are becoming more and more apparent in combinational settings, where the existing expertise within ISCT, combined with an increased presence of stem cell engineering therapies, will help us become a leader in this emerging science.”
The Stem Cell Engineering (SCE) Committee is the newest ISCT Scientific Committee, created as a proactive initiative to promote the clinical translation of therapies involving stem cell engineering, leveraging expertise in Hematopoietic Stem Cells (HSCs) and Immuno and Gene therapies within the society.
Under the leadership of its founding chair, Dr. Jaap Jan Boelens, a medical and scientific leader specialized in bone marrow and cord blood treatments, as well as rare diseases, this committee will establish and steward ISCT positions within the stem cell engineering space, with a focus on driving the clinical translation of engineered stem cells including HSCs, and on the integration of such methods with novel immune and gene therapies. Recognizing that this is an optimal area for increased presence within ISCT, this committee will work to initiate scientific discussion and consolidate expertise in this area within Society membership.
Recognizing that developments in this space are emerging in parallel with new immune and gene therapies, this committee also looks forward to working collaboratively and closely with other ISCT scientific committees, beginning with the Immuno & Gene Therapy Committee, to explore the potential combinational applications of stem cell engineering therapies.

Ivan Martin, PhD
Chair, Mesenchymal Stromal Cell Committee
University of Basel
Basel, Switzerland
“In order to maintain strong leadership, it has become increasingly clear that it is important to tighten connections with both internal ISCT Committees and external international groups to support the challenges of developing and adopting MSC-based treatments.”
The translation of MSCs into clinical trials and products continues to develop on multiple fronts, with manifest interest by the scientific, technical, clinical, regulatory and commercial communities. The MSC Committee has consolidated its position as a vivid forum for discussion of upcoming events and topics in the MSC landscape, and has managed to communicate those internal reflections in the form of published statements or conference sessions.
In order to maintain strong leadership in this specialty, it has become increasingly clear that it is important to enlarge the forum beyond the borders of the Committee. The group has thus tightened connections with other ISCT Committees and opened to a dialogue with other international societies and working groups. We are convinced that – despite being more complex – this is the way to effectively serve the community in increasing awareness on the opportunities and challenges of developing and adopting MSC-based treatments.
2020 has highlighted key issues that remain to be addressed in the translation of MSC research to clinical treatments, including manufacturing and potency considerations. The ISCT MSC Committee continues to develop publications to address such issues, the most recent of which was published in March this year: “Improving mesenchymal stem/stromal cell potency and survival”, and continues to work with the International Standards Organization (ISO) on characterization and general guidelines for biobanking human MSCs

Sandeep Soni, MD
Co-Chair, Immuno & Gene Therapy Committee
CRISPR Therapeutics
San Francisco, USA
“Our committee brings relevant expertise and willing colleagues to identify critical challenges for the rapidly advancing field of immuno & gene therapies. I am excited to see us lead discussions spanning from the science to manufacturing, logistics, and regulation. “
The ISCT Immuno & Gene Therapy Committee is responsible for a prolific and forward-focused effort to bring ISCT member expertise in this field to a consolidated and up-to-date understanding that is the basis for ongoing developments.
In the past year, immuno & gene therapies continue to be a core area in which successful clinical translations are ongoing and in which regulatory approval has already been attained for multiple proven products. From successful CAR-T therapies to leading research in NK-cells, to gene manipulation in both in-vivo and ex-vivo cells, ISCT leaders bring cutting-edge expertise in this specialty to advance scientific discourse. Near the onset of the COVID-19 pandemic, this committee was thus well placed to publish a comparative perspective focused on the viral symptoms of COVID-19 and symptoms targeted by ongoing immuno & gene therapeutic research.
In 2020, we welcome Dr. Sandeep Soni, who brings significant expertise as both an executive leader in industry, and as a researcher and clinician with a focus on ex-vivo gene editing, as co-chair of the Immuno & Gene Therapy Committee.

Joseph Schwartz, MD, MPH
Co-Chair, ISCT Lab Practices Committee
New York – Presbyterian Hospital
New York, USA
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
The ISCT Lab Practices Committee (LPC) is a long-standing committee that has been at the core of ISCT membership since its earliest days. In conjunction with its mandate, the LPC consistently creates key resources and publications aimed at providing ISCT members with the tool they need to build knowledge in their own laboratories and teams. LPC resources provide practical and up-to-date information for member use, and are community-driven. They are made for members, by members.
In 2020, the LPC provided resources dedicated to the development and maintenance of cell therapy production at standards of excellence. From practical guidelines on operations and staffing, to specific steps on the commissioning of clean rooms and conducting process validation, the LPC provided focused and relevant content to address emerging needs for cellular therapy laboratories in particular.
In 2020, we welcome Dr. Joseph Schwartz, a medical specialist and leader in transfusion and cellular therapy, as co-chair of the LPC committee.